Abstract
Objective
To compare the efficacy of statins and ɑ blockers drug therapies for benign prostatic hyperplasia (BPH) in patients with metabolic syndrome (MetS).
Materials and method
A total of three hundred patients were randomly distributed into three groups of one hundred patients each. Group 1 received only ɑ-adrenoceptor antagonist (ɑ-blocker, AB) (Tamsulosin), group 2 received only statin (atorvastatin), and group 3 received AB plus statin (Tamsulosin + Atorvastatin). The efficacy measurement was assessed by analyzing the changes from baseline in the total International Prostate Symptom Score (IPSS), disease-specific QoL question score and maximum urinary flow rate at the end of 6 months in each group and between the three groups.
Results
Pre-treatment and post-treatment value of triglycerides (TG), high-density lipoprotein (HDL), and prostate volüme (PV) were not significantly different in AB group, while TG and PV were significantly lower in patients taking statin and combined therapy. The significant decrease was demonstrated in maximum urinary flow rate (Qmax) in three groups. However, the most significant decrease was observed in the combination therapy group. IPSS, postvoid residual urine volüme (PVR), and Quality of Life score (QoL) significantly changed in three groups.
Conclusion
We recommend of the use of statins in those men with BPH accompanied by MetS in which AB is ineffective alone.
Introduction
Benign prostatic hyperplasia (BPH) is a most common public health disorder in ageing men throughout the world, which can markedly affect quality of life (QoL) [Citation1]. In spite of the high prevalence of BPH, the etiology of BPH is still unclear [Citation2].
Metabolic syndrome (MetS) is an important public health problem worldwide and its prevalence is increasing year by year [Citation3]. MetS is a cluster of chronic disorders including hyperglycemia, dsyslipidemia, hypertension, and visceral obesity. MetS may also cause systemic disorders by affecting adipokines, such as interleukin 18, and testosterone levels [Citation4,Citation5]. In addition, this situation fairly increases the risk of developing cardiovascular diseases and type 2 diabetes mellitus [Citation6]. Although the underlying cause is not entirely clear, recent studies have shown that MetS and its individual components such as dyslipidemia are related to BPH [Citation7–10]. Therewithal, high low-density lipoprotein (LDL) levels have been reported to be associated with increased risk of BPH [Citation11]. Also, Besiroglu et al. showed positive correlation between triglyceride (TG) level and prostate volüme [Citation12,Citation13]. The majority of studies suggest that this condition can be linked to inflammation [Citation14].
Statins are a 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase inhibitor used to improve serum lipid parameters, including reduction in total cholesterol (C), LDL, TG, and increase in high-density lipoprotein (HDL). Thus, statins can reduce cardiovascular morbidity associated with dyslipidemia and vascular disease [Citation15–17]. Through its reduction of intracellular manufacture of mevalonate, a key intermediate in cholesterol biosynthesis, it also reduces isoprenylation of the G-proteins Rho and Ras, which could lead to prostatic smooth muscle relaxation [Citation18]. Statins also inhibit the formation and reduce the levels of cholesterol in the prostate that affects prostate cell growth and survival [Citation19].
In recent studies with the presence of MetS, therapeutic agents used for BPH and lower urinary tract symptoms (LUTS) are shown to have poor activity [Citation20]. This condition suggests that attention should be paid to the presence of MetS in the treatment of LUTS/BPH in daily practice.
In light of these informations, we aimed to compare the efficacy of statins and ɑ blockers drug therapies for LUTS/BPH in patients with MetS.
Methods
This was a multicentre, prospective, randomized study in men who had BPH with MetS. Three hundred men were enrolled in this study who visited our clinic for BPH. All patients underwent baseline evaluation at the initial visit. Investigators took complete medical histories and detailed physical examinations, including weight, height, blood pressure and digital rectal examination (DRE). Transrectal ultrasonography for prostate volume (PV) evaluation was also performed. Body mass index (kg/m2) was calculated as body weight divided by the square of body height. In collaboration with the department of internal medicine, they underwent a serum TG, HDL, LDL, and fasting blood sugar (FBS) examination. Fasting blood samples were taken for biochemical analysis in the morning. Maximum urinary flow rate (Qmax), postvoid residual urine volume (PVR), Quality of Life score (QoL), and International Prostate Symptom Scores (IPSS) were also recorded.
MetS was defined according to the National Cholesterol Education Program’s Third Adult Treatment Panel (NCEP ATPIII) guidelines as the presence of three or more of the following findings: circumference ≥ 90 cm, systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg, TG > 150 mg/dl (1.7 mmol/l), HDL-C < 40 mg/dl (1.03 mmol/l), FBS ≥ 110 mg/dl (6.1 mmol/l), or undergoing treatment for hyperglycemia.
Exclusion criteria included (i) history of previously taking medication for their symptoms within six months or prostate-related surgery; (ii) patients with a suspicion of prostate cancer by DRE or abnormal prostate-specific antigen (PSA) level (> 4 ng/ml); (iii) patients with concomitant urological disorder including urinary tract infection, urethral stricture, urolithiasis or malignancy; and (iv) patients who had a history of neurological disease with bladder dysfunction.
A total of three hundred patients were randomly distributed into three groups of one hundred patients each. In order to reduce potential bias, randomization was done by the head nurse of the outpatient clinic who was blinded to the study. The first subject was randomized January 2015, and the last completed treatment in February 2016. Group 1 received only ɑ-adrenoceptor antagonist (ɑ-blocker, AB) (Tamsulosin), group 2 received only statin (atorvastatin), and group 3 received AB plus statin (Tamsulosin + Atorvastatin).
All cases were visited by urologists third-month and sixth-month and were evaluated again for the response to treatment. The efficacy measurement was assessed by analyzing the changes from baseline in the total IPSS, QoL, and Qmax at the end of 6 months in each group and between the three groups.
The standard effect size was set at 0.93 with 90% effect (1 − β) and 5% standard error (α) margin. According to this calculation, n = 24 cases for each group is sufficient.
Statistical analysis
Paired samples t test was used to compare the outcomes between pre-treatment and post-treatment values in each treatment groups. One-way ANOVA was carried out to compare the mean differences across three groups. The subgroups were compared using Bonferroni post hoc test. All statistical analysis was performed by using SPSS ver. 20.0 (SPSS Inc., Chicago, IL).
Results
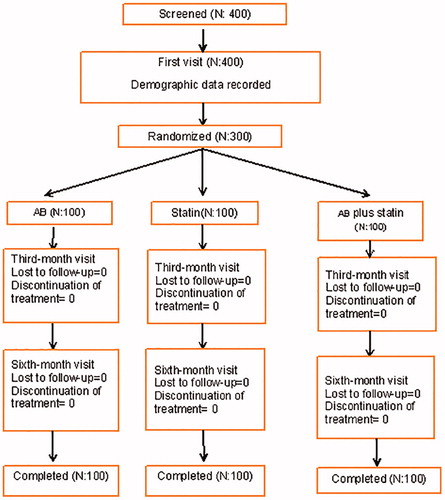
Of 400 patients screened, 300 were enrolled and all of them completed the study (). The patients were divided into three groups according to treatment randomization. Pre-treatment group demographics were similar at baseline and they were demonstrated in . The crude values of post treatment outcomes were also depicted in .
Table 1. The comparison of mean values of outcome measures in three groups.
The parameters were compared for each group and paired differences with confidence intervals were demonstrated in . Pre-treatment and post-treatment value of TG, HDL, and PV were not significantly different in AB group, while TG and PV were significantly lower in patients taking statin and combined therapy. The significant decrease was demonstrated in Qmax in three groups. However, the most significant decrease was observed in the combination therapy group (AB, statin and combined groups Qmax decrease −3.16, −1.55, and −4.74, respectively). IPSS, PVR, and QoL significantly changed in three groups.
Table 2. Paired differences of the outcome measures in three groups.
The treatment groups were compared with each other via outcome measures. Prostate volume was not significantly different in three comparisons. Tamsulosin + Atorvastatin therapy was found superior to both statin and AB therapy with respect to Qmax value. PVR was not significantly different in the comparison of three treatment groups. Tamsulosin + Atorvastatin group revealed more improvement in IPSS value compared with both statin and AB group. The significant difference was observed with respect to QoL between Tamsulosin + Atorvastatin group and other groups, while no significant difference was shown between statin and AB group. All pairwise comparisons were demonstrated in .
Table 3. The comparison of outcome measure across three randomized groups.
Discontinuation due to adverse events was not observed in any patient.
Discussion
ɑ Blockers and are used mostly in the treatment of men LUTS associated with BPH [Citation21]. They are currently considered the first line of standard pharmacological treatment [Citation22]. These drugs disconnect motor sympathetic adrenergic nerve supply to the prostate, reducing urethral resistance and inhibiting smooth muscle tone in the prostate and lower urinary tract, and in this way improving BPH/LUTS [Citation23]. Although numerous evidence has confirmed the effective role of these drugs in BPH/LUTS treatment, the factors affecting their efficacy have still not been completely investigated. A UK study by Kupelian et al. [Citation24] showed that the presence of MetS worsened the symptoms of BPH, including PVR, intermittency, decreased force, and hesitancy, and the overall response to treatment was poor. These results imply that MetS worsens the obstructive symptoms of BPH and also impedes the reduction in symptoms after routine treatment. Also Cyrus et al. showed that MetS negatively affect the response to medical treatment of BPH [Citation20]. The most of observational studies showed that inflammation linked this connection. Really MetS is associated with proinflammatory status and has been associated with prostate inflammation and elevated levels of inflammatory cytokines [Citation10,Citation14].
Beside reducing cholesterol levels, statins have other pharmacological effects, such as anti-inflammatory, procellular adhesion, pro-proliferation of smooth muscle cells, and pro-cell apoptosis effects [Citation25]. Dalaklioglu et al. reported that the diminished relaxation in response to acetylcholine or electrical field stimulation as well as the changes in expression of these proteins in aged rats were significantly improved by pravastatin treatment, and this effect did not seem to be associated with lipid lowering effect of this drug [Citation26]. In addition, an experimental study showed that a high cholesterol diet leads to histological changes in the rat prostate that are similar to those in prostatic hyperplasia [Citation27].
In light of these informations, we evaluated efficacy of statins in the treatment of BPH accompanied by MetS in this comparative study. To our knowledge, this is the first study investigating the effects of AB, statins and their combination in the treatment of the BPH.
In the literature, results of statins in the treatment of BPH are controversial. Zhang et al. demonstrated that simvastatin and atorvastatin treatments are effective in alleviating the symptoms of elderly men with BPH and MetS and that these effects were likely mediated through the lipid-lowering and anti-inflammation effects of the statins. They also showed the reduction in PV was positively related to the decreases in the levels of TG [Citation25]. A retrospective study showed that those taking statins had a lower cumulative incidence of moderate-severe LUTS, a decreased maximum flow rate, and a lower cumulative incidence of BPH compared with those not taking statins [Citation28]. On the other hand, Stamatiou et al. showed that lovastatin treatment does not have any effect on IPSS, PV, and PSA in men with prostatic enlargement due to presumed BPH [Citation29]. In addition, Shih et al. have shown that long-term statin therapy reduces the progression of BPH [Citation30]. According to another study, atorvastatin is not effective in the treatment of men with BPH [Citation31]. However, IPSS, PV, and PSA were evaluated in these studies.
The responders for treatment were defined as those who had a total IPSS decrease and Qmax increase from baseline after six months in this study. In our study; statistical significant IPSS decrease and Qmax increase in all groups but combination therapy gave better results in IPSS and Qmax in patients with MetS. We think that these effects of the statins by through the lipid-lowering and anti-inflammation. The reduction in PV is significant in statin and combination therapy groups. The reduction of PV may be contributed to better results in combination therapy. In contrast to previous studies, we did not evaluated changes in PSA.
Conclusions
We consider that statins effective in the treatment of BPH wits MetS. Thus, we recommend of the use of statins in those men with BPH accompanied by MetS in which AB is ineffective alone. Nevertheless, further large scale and comparative studies are needed in order to obtain more detailed information.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–89.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in america project: benign prostatic hyperplasia. J Urol. 2008;179:S75–S80.
- Gorbachinsky I, Akpinar H, Assimos DG. Metabolic syndrome and urologic diseases. Rev Urol. 2010;12:e157–e180.
- Angelova P, Kamenov Z, Tsakova A, et al. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male. 2018;21:130–137.
- Khripun I, Vorobyev S, Belousov I, et al. Influence of testosterone substitution on glycemic control and endothelial markers in men with newly diagnosed functional hypogonadism and type 2 diabetes mellitus: a randomized controlled trial. Aging Male. 2018;1–9. DOI:10.1080/13685538.2018.1506918
- Eckel RH. Treating dyslipidemia of the metabolic syndrome: where's the evidence?. Nat Rev Endocrinol. 2007;3:437.
- Abdollah F, Briganti A, Suardi N, et al. Metabolic syndrome and benign prostatic hyperplasia: evidence of a potential relationship, hypothesized etiology, and prevention. Korean J Urol. 2011;52:507–516.
- Park YW, Kim SB, Kwon H, et al. The relationship between lower urinary tract symptoms/benign prostatic hyperplasia and the number of components of metabolic syndrome. Urology. 2013;82:674–679.
- Zhang X, Zeng X, Liu Y, et al. Impact of metabolic syndrome on benign prostatic hyperplasia in elderly Chinese men. Urol Int. 2014;93:214–219.
- Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16:101–106.
- Parsons JK, Bergstrom J, Barrett-Connor E. Lipids, lipoproteins and the risk of benign prostatic hyperplasia in community-dwelling men. BJU Int. 2008;101:313–318.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Besiroglu H, Dursun M, Otunctemur A, et al. The association between triglyceride high density lipoprotein cholesterol ratio and benign prostate hyperplasia in non-diabetic patients: a cross-sectional study. Aging Male. 2017;20:198–204.
- Robert G, Descazeaud A, Nicolaiew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate. 2009;69:1774–1780.
- Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696.
- Pedersen TR, Faergeman O, Kastelein JJ, et al. Incremental Decrease in End Points Through Aggressive Lipid Lowering Study G: High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445.
- Ridker PM, Morrow DA, Rose LM, et al. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45:1644–1648.
- Rees RW, Foxwell NA, Ralph DJ, et al. Y-27632, a Rho-kinase inhibitor, inhibits proliferation and adrenergic contraction of prostatic smooth muscle cells. J Urol. 2003;170:2517–2522.
- Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk?. Trends Endocrinol Metab. 2008;19:113–121.
- Cyrus A, Kabir A, Goodarzi D, et al. Impact of metabolic syndrome on response to medical treatment of benign prostatic hyperplasia. Korean J Urol. 2014;55:814–820.
- Lee YC, Liu CC, Juan YS, et al. The impact of metabolic syndrome on the responsiveness to α1-blocker in men with BPH/LUTS . Int J Clin Pract. 2013;67:356–362.
- Chung MS, Lee SH, Park KK, et al. Comparative rapid onset of efficacy between doxazosin gastrointestinal therapeutic system and tamsulosin in patients with lower urinary tract symptoms from benign prostatic hyperplasia: a multicentre, prospective, randomised study. Int J Clin Pract. 2011;65:1193–1199.
- Fulton B, Wagstaff AJ, Sorkin EM. Doxazosin. An update of its clinical pharmacology and therapeutic applications in hypertension and benign prostatic hyperplasia. Drugs. 1995;49:295–320.
- Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol. 2013;189:S107–S114. discussion S115-106.
- Zhang X, Zeng X, Dong L, et al. The effects of statins on benign prostatic hyperplasia in elderly patients with metabolic syndrome. World J Urol. 2015;33:2071–2077.
- Dalaklioglu S, Sahin P, Tasatargil A, et al. Pravastatin improves the impaired nitric oxide-mediated neurogenic and endothelium-dependent relaxation of corpus cavernosum in aged rats. Aging Male. 2014;17:259–266.
- Ploumidou K, Kyroudi-Voulgari A, Perea D, et al. Effect of a hypercholesterolemic diet on serum lipid profile, plasma sex steroid levels, and prostate structure in rats. Urology. 2010;76:1517 e1511–1515.
- St Sauver JL, Jacobsen SJ, Jacobson DJ, et al. Statin use and decreased risk of benign prostatic enlargement and lower urinary tract symptoms. BJU Int. 2011;107:443–450.
- Stamatiou KN, Zaglavira P, Skolarikos A, et al. The effects of lovastatin on conventional medical treatment of lower urinary tract symptoms with finasteride. Int Braz J Urol. 2008;34:555–561. discussion 561-562.
- Shih H-J, Tsai P-S, Wen Y-C, et al. Hyperlipidemia patients with long-term statin treatment are associated with a reduced risk of progression of benign prostatic enlargement. Aging Male. 2018;1–8. DOI:10.1080/13685538.2018.1487392
- Mills IW, Crossland A, Patel A, et al. Atorvastatin treatment for men with lower urinary tract symptoms and benign prostatic enlargement. Eur Urol. 2007;52:503–509.