Abstract
Objective: This study investigated the efficacy of 5-year testosterone replacement therapy (TRT) on lipid profile and glucose tolerance in Japanese hypogonadal men.
Methods: Fourteen patients, who received continuous TRT for 5 years, and 22 controls with 5-year observations were enrolled. The patients in the TRT group had received intramuscular injections of testosterone enanthate (250 mg) every month for 5 years. We collected the following data: blood pressure, fasting blood sugar (FBS), hemoglobin A1c (HbA1c), total cholesterol, triglyceride (TG), high density lipoprotein-Chol values, and prostate specific antigen (PSA) level at baseline, 1-, 3-, and 5-years from initial intervention. These data were compared between the two groups.
Results: There were no statistically significant differences in any other baseline characteristic, excluding SBP, between the two groups. FBS was significantly improved at 3- and 5-year visits in the TRT group compared to the control group. Furthermore, the HbA1c level and TG value demonstrated a significant decrease at 1-, 3-, and 5-years in the TRT group. However, no significant difference in changes to PSA levels from baseline in both groups was observed.
Conclusions: Five-year TRT could improve FBS, HbA1c, and TG levels among Japanese hypogonadal men with no significant increase in PSA.
Introduction
Late-onset hypogonadism (LOH) syndrome presents as various clinical conditions caused by decreasing levels of testosterone in elderly men [Citation1,Citation2]. Testosterone replacement therapy (TRT) has become widely used for the treatment of this syndrome in order to maintain quality of life (QOL) [Citation3–5]. Clinical symptoms include metabolic syndrome, which presents as visceral obesity, hypertension, diabetes mellitus, and dyslipidemia [Citation6,Citation7], and are considered to be a potential risk for cardiovascular disease and cerebral apoplexy. Recent studies have reported success in using TRT to improve these metabolic factors [Citation4,Citation5,Citation8].
Recently, we conducted a randomized controlled trial (RCT; the EARTH study) to investigate the 1-year effect of TRT on the physical and mental health of hypogonadal men in Japan [Citation9]. The EARTH study indicated that 1 year of TRT contributed to decreases in waist circumference and serum triglyceride (TG) levels, but did not result in improvement in blood pressure, glycemic control, total cholesterol (T-Chol) levels, or high density lipoprotein (HDL)-Chol values.
Metabolic syndrome, which is a lifestyle-related disease, is a lifelong disorder correlated with decreased QOL, fatal diseases, and mortality for elderly men. Long-term care might be required for the management of metabolic syndrome. Hence, providing TRT to hypogonadal men is an approach for managing metabolic syndrome and should be continued long-term. Indeed, a recommendation by the International Society for the Study of the Aging Male suggests that men with LOH syndrome should be subject to lifelong monitoring, and long-term TRT is required to achieve maximum QOL benefits [Citation1,Citation2]. However, information about the efficacy of long-term TRT for hypogonadal men is limited. Therefore, we investigated the effects of 5-year TRT on lipid profile and glucose tolerance in Japanese men with LOH syndrome.
Patients and methods
Study patients
Patients (n = 14) who had received continuous TRT for 4 years following the end of the EARTH study were enrolled (. In addition, 22 patients who had undergone observations over 4 years in our hospital were included as controls from the EARTH study.
Figure 1. Flowchart of subjects enrolled from the EARTH study. TRT: testosterone replacement therapy.
A biochemical diagnosis of hypogonadism was based on the Japanese criteria of a free testosterone (FT) level ≤11.8 pg/mL [Citation10]. FT was measured by radioimmunoassay using a DPC Free Testosterone kit (Mitsubishi Kagaku Iatron) in serum collected between 9:00 and 11:00 h at the screening visit for the EARTH study. Exclusion criteria were based on those for the EARTH study. Patients who had been administered a dutasteride, finasteride, anti-androgen agent, or any testosterone supplement during the trial were excluded. The study protocol was approved by the Kanazawa University Hospital Institutional Review Board and all patients gave written informed written consent before participation in the EARTH study.
Study protocol
We collected the following data from the EARTH study database: systolic blood pressure (SBP), diastolic blood pressure (DBP), fasting blood sugar (FBS), hemoglobin A1c (HbA1c), T-Chol, TG, HDL-Chol values, and prostate specific antigen (PSA) level at baseline and the 1-year visit. In addition, we retrospectively collected these parameters at 3- and 5-year intervals after the initial intervention of the EARTH study. These data were compared between the TRT and control groups.
The patients who were assigned to the TRT group had received intramuscular injections of testosterone enanthate (250 mg; Enarmon DepotTM, ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) every month for 5 years. The control group received no testosterone treatment during this trial.
Statistical analysis
The background data from each group were compared using the Mann–Whitney test. Changes in each parameter from baseline were evaluated using the Mann–Whitney test with Bonferroni correction to analyze the efficacy of 5-year TRT. For each group, fluctuations in levels of PSA were compared by Wilcoxon t-test with Bonferroni correction; and variations in each parameter from baseline were evaluated using the Mann–Whitney test with Bonferroni correction. All statistical analyses were performed using SPSS™ version 22 (SPSS Inc., Chicago, IL, USA). In all analyses, p < .05 was considered statistically significant.
Results
The mean ages (±standard deviation [SD]) of patients in the TRT and control groups were 62.6 ± 7.8 and 66.1 ± 8.4 years, respectively (). The mean (SD) FT values in the TRT and control groups were 7.90 ± 1.91 and 7.30 ± 1.92 pg/dl, respectively. In the data available from the background patients, SBP was significantly higher in the control than TRT group (122 ± 10 vs. 135 ± 17 mmHg, p = .0171). There were no statistically significant differences in any other baseline characteristics between the two groups.
Table 1. Comparisons of background characteristics in TRT and control groups.
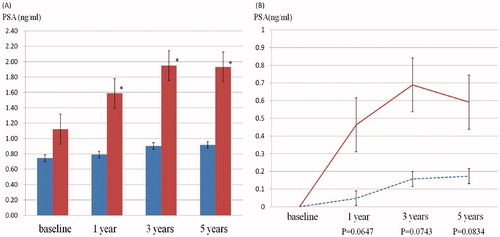
Comparison of the changes in each parameter in both groups showed that variations from baseline to 3- and 5-years in FBS value were significantly decreased in the TRT group compared with the control group (. Furthermore, there were significant decreases in HbA1c level and TG value at the 1-, 3-, and 5-year visits in the TRT group. No significant alterations from baseline in measures of SBP, DBP, T-Chol, and HDL-Chol were seen at each visit in either group. The level of PSA did not significantly vary between each visit in the TRT group. The level of PSA was significantly higher in the control group, but within the normal reference range, and there were no significant changes in level of PSA from baseline between the two groups (. No patients were given a diagnosis of prostate cancer or had worsening voiding symptoms.
Figure 2. Comparison of the changes in each parameter from baseline in TRT and control groups. Significant improvement in FBS was observed at the 3- and 5-year visits. Moreover, the HbA1c level and TG value showed significant decreases at 1-, 3-, and 5-years. Border line with blue (TRT group); Straight line with red (control group); * significant difference; SBP: systolic blood pressure; DBP: diastolic blood pressure; FBS: fasting blood sugar; HbA1c: hemoglobin A1c; Tchol: total cholesterol; TG: triglyceride; HDL-Chol: high density lipoprotein-cholesterol.
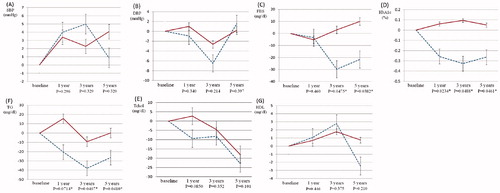
Figure 3. Changes in PSA levels for TRT (blue) and control (red) groups. Mean levels of PSA in TRT and control groups at 1-, 3-, and 5-years are indicated. In the control group, PSA levels showed a significant increase at each visit compared to the baseline level (* significant changes, p < 0.05). However, the TRT group showed no significant change in PSA level at any visit (A). The changes in PSA level from baseline in both groups were compared. There was no significant change in both groups (B).
Discussion
This is the first longitudinal study undertaken to investigate the efficacy of 5-year TRT on lipid profile and glucose tolerance in Japanese men. In addition, this is a case control study comprising patients receiving TRT and controls who had been randomly assigned in the EARTH study. This study demonstrates that 5-year TRT may contribute to significant improvement in FBS value, HbA1c level, and TG value among Japanese hypogonadal men.
It is widely accepted that various metabolic factors, such as obesity, hypertension, dyslipidemia, and insulin resistance, are associated with testosterone deficiency [Citation4,Citation6,Citation7]. In addition, some previous longitudinal studies have demonstrated the prospective association of testosterone deficiency with the incidence of various lifestyle-related diseases. Men with testosterone levels in the lower quarter of the measured range are likely to have an approximate 2-fold higher risk of developing metabolic syndrome and diabetes than other men [Citation10–12]. One previous study described low levels of testosterone as being significantly correlated with increases of intra-abdominal fat and visceral obesity after 7 years [Citation10]. Another longitudinal study that included 702 middle-aged men with 11 years of follow-up found that lower total testosterone resulted in a risk of developing metabolic syndrome and diabetes mellitus, with odds ratios (OR) of 2.3 (95% CI; 1.5–3.4) and 2.3 (95% CI; 1.3–4.1), respectively [Citation11]. A Massachusetts population-based study that included a random sample of 1156 men aged 40–70 years revealed that low levels of testosterone resulted in a risk of developing type 2 diabetes with an OR of 1.58 for a decrease of 1 SD in free testosterone [Citation12]. Hypogonadism is significantly associated with future development of several clinical conditions, which may lead to fatality, and therefore long-term interventions are likely to be required.
Many previous studies state that TRT can improve body composition, metabolic parameters, and the symptoms of metabolic syndrome in hypogonadal men [Citation1,Citation2,Citation4,Citation5,Citation8,Citation13]. However, a consensus regarding the period of time over which TRT should be administered has not yet been reached. As described above, many lifestyle-related diseases, including various metabolic conditions, are lifelong and hence clinical interventions are often required for the remainder of the patient’s life. If TRT is offered to assist in the management of these conditions, it should be provided long-term.
Recent trials have supported the long-term use of TRT to obtain beneficial effects for various metabolic parameters and hypogonadal symptoms. Francomano et al. reported that 5-year TRT with 1,000 mg T-undecanoate injections every 12 weeks in 20 hypogonadal men contributed to a significant reduction in waist circumference and improvements in glycemic control, lipid profile, SBP, and DBP compared to 20 matched non-TRT controls [Citation14]. A separate longitudinal study demonstrated that TRT given using parenteral T-undecanoate at 1,000 mg every 12 weeks for 60 months improved all metabolic parameters including T-Chol, LDL-Chol, TG, HDL-Chol, SBP, DBP, FPG, and HbA1c in 255 men with mild symptoms of LOH [Citation15]. Interestingly, that study also determined that C-reactive protein levels, which are known to predict cardiovascular events [Citation16], decreased through the use of TRT, suggesting that TRT could decrease the risk of cardiovascular disease by improving various metabolic factors. Another study of 185 hypogonadal men declared that 4-year TRT showed benefits in glycemic control, weight, waist circumference, and erectile function [Citation17]. The results of the present study are likely to be similar to those reported previously in confirming that long-term TRT may also be beneficial for lipid profile and glucose tolerance among Japanese hypogonadal men.
In the present study, the fluctuations in levels of PSA were within the normal range and there was no significant difference in changes of PSA levels from baseline between visits among the two groups. This finding suggests that 5-year TRT may have no impact on the level of PSA and is not associated with a risk of developing of prostate cancer. A previous study demonstrated that 5-year TRT resulted in a significant increase in PSA levels within the normal range. This rise occurred within the first 12 months of treatment and remained stable for the rest of the study period [Citation14]. Another report demonstrated that 5-year TRT led to a slight increase in PSA levels and prostate volume, but not to increased risk of worsening urinary symptoms and incidence of prostate cancer [Citation15]. Moreover, safety and efficacy in delivering TRT at 12-week intervals for a maximum of 11 years has recently been reported [Citation18]. Some recent studies suggest some beneficial effects of TRT on urinary functions [Citation19–21]. Therefore, long-term TRT is likely to be well tolerated by patients and safe to use. However, in the EARTH study, patients with a baseline PSA level of over 2.0 ng/ml or severe voiding symptoms were eliminated; therefore, further studies that include patients with various levels of PSA and voiding symptoms are necessary to reach more definite conclusions regarding the long-term safety of TRT among Japanese hypogonadal men.
There are certain limitations to the present study. First, this study comprised a small number of subjects. Next, there is a population bias, especially in the TRT group. As patients in the TRT group may have experienced benefits from the therapy, they may have been biased toward continuing the treatment for 5 years. Additionally, many participants received other internal medicine interventions and were administered with treatments including antihypertensive drugs, lipid-lowering drugs, and anti-diabetic medications. Therefore, some metabolic parameters may have been affected by these biases. Moreover, patients receiving TRT might have greater motivation to improve their metabolic condition. However, the present report lacked information about patient exercise and eating habits. On the other hand, the study included subjects that had been assigned at random to TRT and control groups and comparisons between the two groups strengthen our results. Further prospective randomized studies to include more participants and long-term observations are required to corroborate the results of this study.
In conclusion, 5-year TRT may improve FBS, HbA1c, and TG levels among Japanese hypogonadal men with no significant increase in PSA.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18:5–15.
- Morales A, Lunenfeld B, International Society for the Study of the Aging Male. Investigation, Treatment and Monitoring of Late-Onset Hypogonadism in Males. Official Recommendations of ISSAM. Aging Male 2002;5:74–86.
- Lunenfeld B, Arver S, Moncada I, et al. How to help the aging male? Current approaches to hypogonadism in primary care. Aging Male. 2012;15:187–197.
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009;5:427–448.
- Shigehara K, Konaka H, Nohara T, et al. Effects of testosterone replacement therapy on metabolic syndrome among Japanese hypogonadal men: A subanalysis of a prospective randomised controlled trial (EARTH study). Andrologia. 2018;50. DOI:10.1111/and.12815
- Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male. 2016;19:85–89.
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male. 2014;17:161–165.
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169.
- Konaka H, Sugimoto K, Orikasa H, et al. Effects of long-term androgen replacement therapy on the physical and mental statuses of aging males with late-onset hypogonadism: a multicenter randomized controlled trial in Japan (EARTH Study). Asian J Androl. 2016;18:25–34.
- Tsai EC, Boyko EJ, Leonetti DL, et al. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes. 2000;24:485–491.
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–1041.
- Stellato RK, Feldman HA, Hamdy O, et al. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts Male Aging Study. Diabetes Care. 2000;23:490–494.
- Dandona P, Dhindsa S, Chaudhuri A, et al. Hypogonadotrophic hypogonadism in type 2 diabetes. Aging Male. 2008;11:107–117.
- Francomano D, Lenzi A, Aversa A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int J Endocrinol. 2014;2014:527470.
- Traish AM, Haider A, Doros G, et al. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68:314–329.
- Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. Aging Male. 2016;19:239–243.
- Hackett G, Cole N, Mulay A, et al. Long-term testosterone therapy in type 2 diabetes is associated with decreasing waist circumference and improving erectile function. World J Mens Health. 2018. DOI:10.5534/wjmh.180052M
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–246.
- Karazindiyanoğlu S, Cayan S. The effect of testosterone therapy on lower urinary tract symptoms/bladder and sexual functions in men with symptomatic late-onset hypogonadism. Aging Male. 2008;11:146–149.
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14:53–58.
