Abstract
Objective
The association between asthma and benign prostatic hyperplasia (BPH) has rarely been explored. We investigated whether male asthmatic patients had an increased risk of BPH by conducting this retrospective nationwide population-based study.
Methods
We utilized data derived from the National Health Insurance Research Database (NHIRD) in Taiwan. A total of 9778 male patients aged >40 years who were newly diagnosed with asthma between 2000 and 2006 were included in the asthma group. Male enrollees without asthma were selected as the non-asthma group from the same database. Both the groups were followed up until the end of 2013. We performed Cox proportional hazard regression analysis to estimate the risk of BPH and transurethral resection of the prostate (TURP) in the male patients with asthma compared with that in those without asthma.
Results
The risk of BPH and TURP in the asthma group was 1.40-fold (95% confidence interval [CI] = 1.30–1.42) and 1.30-fold (95% CI= 1.31–1.50) higher than that in the non-asthma group, respectively, after adjusting for comorbidities, relevant medications and number of annual outpatient visits.
Conclusions
The male patients with asthma were found to have a higher risk of BPH than did those without asthma.
Introduction
Asthma and benign prostatic hyperplasia (BPH) are two of the most prevalent health problems worldwide. More than 235 million people are estimated to be affected by asthma globally [Citation1]. The prevalence of BPH is approximately 25.3% in men aged between 40 and 79 years, and it increases to >50% in men aged ≥60 years [Citation2,Citation3]. Both asthma and BPH exert a substantial adverse effect on patients’ quality of life, health service systems, and society [Citation4–6].
Patients with asthma usually experience shortness of breath, coughing, and wheezing. They are also reported to have a higher risk of nonrespiratory disorders, such as rheumatoid arthritis, endometriosis, and inflammatory bowel disease, compared with those without asthma [Citation7–9].
Although the contribution of androgens to BPH development is well-known [Citation10], the prevalence of BPH is actually higher in older men, whose androgen levels decline with age [Citation11], indicating that other risk factors might be associated with the pathogenesis of BPH. In addition to aging, other reported health-related risk factors are obesity, metabolic syndrome, and sedentary lifestyle [Citation12,Citation13].
Asthma is characterized by smooth muscle hypertrophy and chronic inflammation of the airway. Emerging evidence suggests that chronic inflammation of the prostate can play a crucial role in the pathogenesis and progression of BPH [Citation14–16]. However, few studies have focused on the relationship between asthma and BPH. Therefore, in this study, we evaluated whether male asthmatic patients have a higher risk of BPH compared with those without asthma by conducting a retrospective cohort study by using data retrieved from the Taiwan National Health Insurance Research Database (NHIRD).
Methods
Data source
The Taiwan government implemented the compulsory, single-payer National Health Insurance (NHI) program in 1995, and the program now covers >99% of the population in Taiwan. The NHIRD contains data regarding beneficiaries enrolled in the NHI. In this study, we used the Longitudinal Health Insurance Database 2000 (LHID2000), which is a subset of the NHIRD. The LHID2000 contains the medical reimbursement claims data of 1 million enrollees for the period between 1996 and 2013 along with detailed information concerning the demographics, clinical visit dates, prescriptions, and diagnostic codes of each enrollee. The NHI administration reported no differences in the distributions of sex and age between patients in the LHID2000 and those in the NHIRD. Therefore, the LHID2000 is considered to be highly representative of the general population. To protect enrollees’ privacy and security, all identification data are encrypted before any data is released for research purposes.
Study population
We designed this study as a retrospective population-based cohort study. We identified male patients who were newly diagnosed with asthma between January 1 2000 and December 31 2006 and included them in the asthma group. Patients with asthma were defined as male patients aged ≥40 years who were assigned the International Classification of Diseases, ninth edition, Clinical Modification (ICD-9-CM) code 493.xx and were prescribed the corresponding medications (inhaled corticosteroids, systemic [oral or intravenous] corticosteroid, inhaled short-acting beta-2 agonists, or long-acting beta-2 agonists). To further validate the diagnosis of asthma, only patients with at least three outpatient (OPD) or ever hospitalization for asthma were included. The date of first diagnosis with asthma was defined as the index date. For each patient with asthma, four enrollees without a diagnosis of asthma, who were frequency matched for age (every 5 years) and the index year, were selected and included in the non-asthma group. Any enrollees who were younger than 40 years and had a history of BPH were excluded from both the groups.
The primary outcome measure of interest was BPH, which was defined as at least three OPD visits or ever hospitalization with a diagnosis code of BPH (ICD-9-CM code 600.0). All enrollees in both the groups were followed up from the index date until the end of 2013, the first date of BPH diagnosis, or withdrawal from the NHI, whichever occurred first. The secondary outcome was transurethral resection of the prostate (TURP), defined by the NHI reimbursement codes 79406B (resection prostate chips more than 5 g), 79411B (resection prostate chips 15–50 g), and 79412B (resection prostate chips> 50 g). Demographic variables considered were age (age groups: 40–54, 55–69, and ≥70 years) and the number of OPD visits. Comorbidities considered were diabetes mellitus (DM, ICD-9-CM code 250), dyslipidemia (ICD-9-CM code 272), hypertension (ICD-9-CM codes 401–405), and heart failure (ICD-9-CM code 428). All comorbidities were determined before the index date. We also considered long-term users of tiotropium and ipratropium if they were prescribed these medications during the follow-up period.
Statistical analysis
Continuous variables are presented as the mean and standard deviation (SD) and categorical variables as the frequency and percentage. Student’s t-test and the chi-square test were used to compare continuous and categorical variables, respectively, between the groups. We calculated the incidence rate of BPH (per 1000 person-years) as the number of BPH cases divided by person-time at risk for both asthma and non-asthma groups. The Kaplan–Meier method was used to determine the cumulative incidence of BPH, and the log-rank test was used to examine significant differences between the groups during the follow-up period. We used univariate and multivariate Cox proportional hazard regression models to determine the hazard ratios (HRs) and 95% confidence intervals (CIs) of BPH associated risk factors and the incidence of BPH. The multivariate model was adjusted for age, DM, dyslipidemia, hypertension, heart failure, tiotropium use, ipratropium use, and the number of annual OPD visits. We also investigated the association between asthma and BPH risk in various subgroups according to age and comorbidity. All statistical analyses were performed using SAS version 9.4 software (SAS Institute, Inc., Cary, NC), and two-tailed p<.05 was considered significant.
Results
Overall, we included 9778 male patients with asthma and 39,112 enrollees without asthma (). The mean age was approximately 63 years in both the groups and the distribution of age did not differ significantly between the groups. Compared with the non-asthma group, the asthma group had a significantly higher prevalence of DM, dyslipidemia, hypertension, heart failure, and relevant medication use. The mean number of annual OPD visits was 35.0 (SD= 24.0) and 20.9 (18.3) in asthma and non-asthma groups, respectively.
Table 1. Comparison of baseline demographic factors and comorbidities between the study patients with and without asthma.
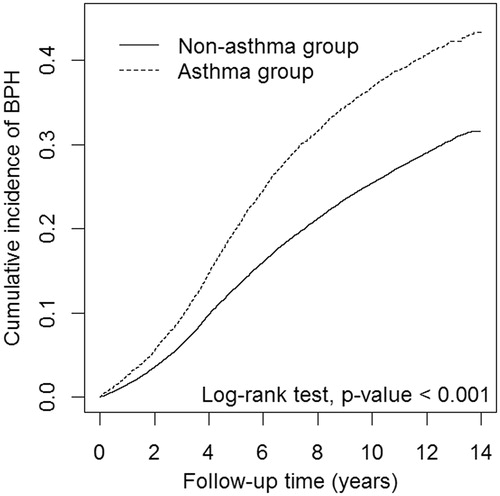
During the mean follow-up period of 8.02 years, 3212 patients in the asthma group and 9062 enrollees in the non-asthma group developed BPH, with an incidence rate of 43.9 and 28.4 per 1000 person-years, respectively. presents cumulative incidence curves for both the groups. We found that the cumulative incidence of BPH was significantly higher in the asthma group than it was in the non-asthma group (log-rank test, p<.001).
Figure 1. Cumulative incidence curves of benign prostatic hyperplasia for groups with and without asthma. BPH: benign prostatic hyperplasia.
The findings of the Cox proportional hazard regression model revealed that the patients with asthma had a higher risk of BPH than did those without asthma, after adjustment for age, comorbidities, medications used, and the number of annual OPD visits (adjusted HR =1.40, 95% CI =1.30–1.42; ). The patients in the age groups of ≥70 and 55–69 years exhibited a 4.01-fold (95% CI =3.78–4.24) and 2.74-fold (95% CI =2.59–2.89) higher adjusted HR for BPH than did the patients in the age group of 40–54 years. Moreover, the patients with dyslipidemia, hypertension, heart failure, long-term ipratropium use, and ≥25 annual OPD visits had a significantly increased risk of BPH.
Table 2. Cox model-measured hazard ratios and 95% confidence intervals of BPH associated with asthma and covariates.
lists the incidence rate and HRs of BPH according to the asthma status stratified by age and comorbidity. We found that the patients with asthma had a significantly higher risk of BPH than did those without asthma. The adjusted HRs of BPH were 1.41 (95% CI =1.26–1.58), 1.37 (95% CI =1.28–1.46), and 1.17 (95% CI =1.10–1.25) in the age groups of 40–54, 55–69, and ≥70 years, respectively. In each comorbidity stratification, the association between asthma and BPH remained similar.
Table 3. Incidence rate and hazard ratios of BPH for the effect of asthma stratified by age and comorbidities.
presents the incidence rate and HRs of TURP according to asthma status and stratified by age. Patients with asthma had a significantly higher incidence of receiving TURP than did those without asthma. Overall, the adjusted HRs of TURP were 1.30 (95% CI =1.13–1.50), when stratified by age, the HR remained significant in age 55–69 (adjusted HR =1.38, 95% CI =1.12–1.68).
Table 4. Incidence and hazard ratios of transurethral resection of the prostate between nonasthma and asthma group.
Discussion
The results of this large population-based cohort study revealed that the male asthmatic patients were 1.40-fold more likely to develop BPH compared with those without asthma after adjusting for age, comorbidities, relevant medications, and number of annual OPD visits. The findings of stratified analysis showed that the risk of BPH remained significantly higher in all age groups beyond 40 years. Moreover, asthmatic patients were 1.30-fold more likely to receive TURP compared with those without asthma. To the best of our knowledge, this is the first study to report that male patients with asthma have an increased risk of BPH compared with those without asthma.
Although few studies have indicated the relationship between asthma and BPH, several lines of evidence can offer possible explanations. Asthma is characterized by smooth muscle hypertrophy and chronic inflammation of the airway, resulting from complex interactions of multiple cells, cytokines, and hundreds of molecular pathways [Citation17,Citation18]. Similarly, various proinflammatory cytokines, growth factors, and chemokines were reported to be upregulated in the prostatic tissues of patients with BPH [Citation19,Citation20], implying that asthma and BPH may have shared underlying pathogenesis mechanisms. For example, an elevated interleukin (IL)-17 level was observed in activated T cells and in epithelial and smooth muscle cells in BPH [Citation19,Citation21], which in turn stimulates the production of stromal growth promoters, namely IL-6 and IL-8, in BPH [Citation19,Citation20,Citation22]. Likewise, a series of studies have suggested the involvement of IL-17 in multiple aspects of asthma pathogenesis, such as structural alterations of epithelial cells and contraction of the smooth muscle [Citation23–25]. The transcription factor hypoxia-inducible factor-1α, which is induced by proinflammatory cytokines and involved in prostate hyperplasia under inflammatory conditions [Citation26], has also been reported to serve as a crucial inflammatory regulator in the allergic inflammation, airway hyperresponsiveness, and pathogenesis of asthma [Citation27,Citation28].
In this study, we included DM, dyslipidemia, hypertension, and heart failure as comorbidities for adjustment in the analysis because previous studies have suggested an association of obesity, DM, dyslipidemia, and hypertension with an increased risk of BPH [Citation29–31]. Our results indicated that the prevalence of dyslipidemia, hypertension, and heart failure was higher in the asthma group than in the non-asthma group (). Furthermore, the patients with dyslipidemia, hypertension, and heart failure had a significantly higher risk of BPH than did those without these comorbidities (); this finding is generally consistent with those of previous studies. Because obesity is highly associated with DM, dyslipidemia, and hypertension [Citation32], we did not further include obesity as comorbidity for adjustment to prevent bias in the results. The number of annual OPD visits appeared to be higher in the asthma group () and was associated with a higher BPH risk (). Therefore, we also included the number of annual OPD visits as a covariate in the analysis to minimize this potential surveillance bias.
Patients with asthma might occasionally misdiagnose as chronic obstructive pulmonary disease (COPD), or present as asthma-COPD overlap, thus prescribed with inhaled anticholinergic agents for symptom control. A causal relationship might be present between inhaled anticholinergic agents and acute urinary retention [Citation33,Citation34]. In other words, inhaled anticholinergic agent use might increase the risk of a patient being diagnosed with BPH. To minimize this potential confounding effect in our study, we included two commonly used anticholinergic agents, namely ipratropium and tiotropium, as covariates for adjustment. Our data showed that long-term use of tiotropium did not alter the risk of BPH, which was consistent with the results of a previous study [Citation35]. By contrast, although not statistically significant, the long-term use of ipratropium tended to be associated with a higher risk of BPH. We speculate that differences between tiotropium and ipratropium in terms of BPH risk may at least be partially attributable to different types of medication administration. Specifically, medication administered through nebulizers may exert more systemic effects compared with a dry powder inhaler and metered dose inhaler (MDI). In our study, ipratropium was administered through a nebulizer or MDI, whereas tiotropium was not administered through a nebulizer. Therefore, ipratropium might have exerted more profound systemic effects compared with tiotropium. This interpretation is supported by a nested case–control study conducted by Afonso et al. [Citation34] who suggested that acute urinary retention was positively associated with the current use of anticholinergic agents, especially in patients with BPH, or administration of anticholinergic agents through a nebulizer.
In the stratified analysis by age (), although the incidence of BPH was found to increase with age, the increased risk of BPH in the patients with asthma appeared to decline with age compared with in those without asthma. This may be because the number of comorbidities usually increased with the patients’ age; therefore, interactions among these comorbidities might have reduced the effect of asthma on BPH risk.
In the NHI program, TURP is indicated for patients with complications induced by BPH or urinary symptoms, and refractory to medical treatment. Therefore, we further investigate whether asthmatic male patients were at a higher risk of intensive surgical treatment of BPH. We found that the risk of TURP is 1.30-fold higher in male patients with asthma compared with those without asthma, particularly in patients aged between 40 and 59 years (). This observation not only strengthens our main finding that asthma is associated with BPH, but also provides more clinical significance for clinicians.
The strength of this retrospective population-based study is that the data used are highly representative of the general population because the NHI is a compulsory program that covers >99% of Taiwan’s residents. However, this study has some limitations that should be addressed. First, each diagnosis in the study was based on ICD-9-CM codes retrieved from administrative data, and detailed clinical information – such as pulmonary function test findings, prostate volume, and serum prostate-specific antigen level of each patient – was unavailable. Thus, determining the severity and phenotype of both asthma and BPH was difficult, and we could not determine whether patients with more severe asthma have even higher incidence or more severe BPH, either. Second, because data in the NHIRD are all encrypted to protect privacy, information regarding the lifestyle of each participant, such as cigarette smoking, dietary preference, and physical activity, were lacking, which may have biased our study results. Last, although we designed the study meticulously, unknown confounding factors may still exist. Despite these limitations, given the large sample size, long period of follow-up, and validity of diagnosis in the study, we believe that the relationship between asthma and BPH identified herein remains highly reliable.
In conclusion, our study results suggest that male patients with asthma aged ≥40 years have an increased risk of BPH compared with those without asthma. Clinicians should be aware of this phenomenon. Future studies can focus on the different phenotypes of asthma and BPH and the underlying pathophysiology of these two diseases.
Ethical approval
The Institutional Review Board of China Medical University Hospital approved this study [CMUH104-REC2-115(CR-3)].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. [cited 2018 Sep 17]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/asthma
- Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471.
- Thorpe A, Neal D. Benign prostatic hyperplasia. Lancet. 2003;361:1359–1367.
- Ford ES, Mannino DM, Homa DM, et al. Self-reported asthma and health-related quality of life: findings from the behavioral risk factor surveillance system. Chest. 2003;123:119–127.
- Calogero AE, Burgio G, Condorelli RA, et al. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male. 2018;1–8. DOI:10.1080/13685538.2018.1434772
- Erkoc M, Otunctemur A, Besiroglu H, et al. Evaluation of quality of life in patients undergoing surgery for benign prostatic hyperplasia. Aging Male. 2018;21:238–242.
- Rolfes MC, Juhn YJ, Wi CI, et al. Asthma and the risk of rheumatoid arthritis: an insight into the heterogeneity and phenotypes of asthma. Tuberc Respir Dis. 2017;80:113–135.
- Peng YH, Su SY, Liao WC, et al. Asthma is associated with endometriosis: a retrospective population-based cohort study. Respir Med. 2017;132:112–116.
- Kuenzig ME, Barnabe C, Seow CH, et al. Asthma is associated with subsequent development of inflammatory bowel disease: a population-based case-control study. Clin Gastroenterol Hepatol. 2017;15:1405–1412 e3.
- Asiedu B, Anang Y, Nyarko A, et al. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male. 2017;20:17–22.
- McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12:S122–S128.
- Besiroglu H, Dursun M, Otunctemur A, et al. The association between triglyceride high density lipoprotein cholesterol ratio and benign prostate hyperplasia in non-diabetic patients: a cross-sectional study. Aging Male. 2017;20:198–204.
- Besiroglu H, Ozbek E, Dursun M, et al. Visceral adiposity index is associated with benign prostatic enlargement in non-diabetic patients: a cross-sectional study. Aging Male. 2018;21:40–47.
- Hu J, Zhang L, Zou L, et al. Role of inflammation in benign prostatic hyperplasia development among Han Chinese: a population-based and single-institutional analysis. Int J Urol. 2015;22:1138–1142.
- Gandaglia G, Briganti A, Gontero P, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013;112:432–441.
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216.
- Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–584.
- Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386:1086–1096.
- Steiner GE, Newman ME, Paikl D, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182.
- Ou Z, He Y, Qi L, et al. Infiltrating mast cells enhance benign prostatic hyperplasia through IL-6/STAT3/Cyclin D1 signals. Oncotarget. 2017;8:59156–59164.
- Arivazhagan J, Nandeesha H, Dorairajan LN, et al. Association of elevated interleukin-17 and angiopoietin-2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118.
- Castro P, Xia C, Gomez L, et al. Interleukin-8 expression is increased in senescent prostatic epithelial cells and promotes the development of benign prostatic hyperplasia. Prostate. 2004;60:153–159.
- Chesne J, Braza F, Mahay G, et al. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–1101.
- Hall SL, Baker T, Lajoie S, et al. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol. 2017;139:462–471 e14.
- Camargo LDN, Righetti RF, Aristoteles L, et al. Effects of anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol. 2017;8:1835.
- Kim HJ, Park JW, Cho YS, et al. Pathogenic role of HIF-1alpha in prostate hyperplasia in the presence of chronic inflammation. Biochim Biophys Acta. 2013;1832:183–194.
- Dewitz C, McEachern E, Shin S, et al. Hypoxia-inducible factor-1alpha inhibition modulates airway hyperresponsiveness and nitric oxide levels in a BALB/c mouse model of asthma. Clin Immunol. 2017;176:94–99.
- Huerta-Yepez S, Baay-Guzman GJ, Bebenek IG, et al. Hypoxia inducible factor promotes murine allergic airway inflammation and is increased in asthma and rhinitis. Allergy. 2011;66:909–918.
- Gacci M, Corona G, Vignozzi L, et al. Metabolic syndrome and benign prostatic enlargement: a systematic review and meta-analysis. BJU Int. 2015;115:24–31.
- Shih HJ, Huang CJ, Lin JA, et al. Hyperlipidemia is associated with an increased risk of clinical benign prostatic hyperplasia. Prostate. 2018;78:113–120.
- Hwang EC, Kim SO, Nam DH, et al. Men with hypertension are more likely to have severe lower urinary tract symptoms and large prostate volume. Low Urin Tract Symptoms. 2015;7:32–36.
- Kyrou I, Randeva HS, Weickert MO, et al. Clinical problems caused by obesity. In: De Groot LJ, Beck-Peccoz P, Chrousos G, et al. editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000.
- Loke YK, Singh S. Risk of acute urinary retention associated with inhaled anticholinergics in patients with chronic obstructive lung disease: systematic review. Ther Adv Drug Saf. 2013;4:19–26.
- Afonso AS, Verhamme KM, Stricker BH, et al. Inhaled anticholinergic drugs and risk of acute urinary retention. BJU Int. 2011;107:1265–1272.
- Miyazaki H, Suda T, Otsuka A, et al. Tiotropium does not affect lower urinary tract functions in COPD patients with benign prostatic hyperplasia. Pulm Pharmacol Thers. 2008;21:879–883.
