Abstract
Objective
We performed this meta-analysis to assess serum testosterone changes in adult males with Type 2 diabetes (T2DM).
Methods
PubMed, Embase, Web of Science, and Cochrane Library were searched to identify qualified studies. Pooled weighted mean differences (WMDs) with 95% confidence intervals (CIs) were utilized to test the changes of total testosterone (TT), free testosterone (FT) and sex hormone-binding globulin (SHBG) in patients with T2DM. Besides, trial sequential analysis was used to verify the pooled results.
Results
A total of 56 studies were enrolled in our meta-analysis. Meta-analyses of the cross-sectional studies showed that patients with T2DM has significant decreases in TT (WMD: −2.98, 95%CI: −3.48 to −2.47), FT (WMD: −32.82, 95%CI: −39.70 to −25.95) and SHBG (WMD: −2.47, 95%CI: −3.93 to −1.02). In terms of the prospective studies, our results showed decreases in TT (WMD: −2.35, 95%CI: −3.24 to −1.46), FT (WMD: −25.96, 95%CI: −83.98 to 32.05), and SHBG (WMD: −10.06, 95%CI: −13.29 to −6.84) in patients with T2DM. By trial sequential analyses, the findings in current meta-analysis were based on reliable evidence.
Conclusion
Our results indicate that patients with T2DM have lower serum TT, FT, and SHBG levels.
Introduction
Type 2 diabetes mellitus (T2DM), characterized by high blood sugar, insulin resistance, and relative lack of insulin, accounts for at least 90% of all cases of diabetes. According to the International Diabetes Federation (IDF), more than 415 million adults have diabetes across the globe, and by 2040, it is estimated that 642 million adults will have diabetes because of population growth, ageing, physical inactivity and increased prevalence of obesity. There are a number of health problems or medications that can predispose to diabetes. Some of the problems or medications include hypoandrogenism, glucocorticoids, thiazides, and statins [Citation1–4].
Testosterone is the primary male sex hormone and an anabolic steroid. In males, testosterone plays an important role in the development of male reproductive tissues such as penis, testes and prostate, as well as promoting secondary sexual characteristics. In addition, testosterone is involved in health and well-being, and the prevention of osteoporosis [Citation5,Citation6]. Insufficient levels of testosterone in men may lead to abnormalities including erectile dysfunction, lower urinary tract symptoms, inflammatory diseases and increased cardiovascular risk factors [Citation7–9]. The corresponding is that testosterone supplement therapy can improve glycemic control, urinary and sexual function, voiding symptoms, and quality of life [Citation10–15]. Although the importance of testosterone in maintaining cardiovascular health is still controversial [Citation16], maintaining normal testosterone levels in elderly men has been shown to improve many parameters that are thought to reduce cardiovascular disease risk, such as increased lean body mass, decreased visceral fat mass, decreased total cholesterol, and glycemic control [Citation8].
The association between T2DM and testosterone has been studied in various studies. Hypoandrogenism has been linked with glycometabolism including insulin resistance, adiposity, and using of glucocorticoids according to many studies [Citation17,Citation18]. Since early 1980s, many researches have focused on the role of testosterone in T2DM and have explored the differences of serum total testosterone (TT), free testosterone (FT), sex hormone-binding globulin (SHBG) between patients with T2DM and participants without T2DM [Citation19]. In most studies, patients with T2DM have significant lower serum TT, FT, and SHBG levels. However, some studies showed non-significant results or contradictory results. For instance, Bai et al. showed no significant differences of serum TT levels between T2DM and non-T2DM participants [Citation20], while Achemlal et al. demonstrated a significant higher TT levels in T2DM patients [Citation21]. However, the results might own to limited sample size or other potential bias. Meta-analysis is a powerful tool which can provide more convinced results than any single study, especially in explaining controversial conclusions. Previous meta-analyses have explored the association between testosterone and type 2 diabetes by pooling data from articles published before 2010 [Citation19,Citation22]. However, lack of subgroup analyses is a limitation in these meta-analyses. Furthermore, large amounts of articles have studied this association from 2010 to 2017 [Citation20,Citation23–45], and the sample size almost doubled in these years. An updated meta-analysis is necessary to provide more information of the association between testosterone and T2DM.
In the current meta-analysis, we searched all cross-sectional and prospective studies and performed this meta-analysis to assess serum testosterone changes in adult males with T2DM and evaluate the role of testosterone in the etiology of T2DM. All relevant information including participant’s age, body mass index (BMI), ethnicity, and erectile function was extracted to get a more detailed evaluation of testosterone in T2DM patients. Furthermore, trial sequential analysis (TSA) was first conducted for comprehensively assessing the changes of serum testosterone in T2DM patients.
Methods
The current meta-analysis was strictly performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [Citation46].
Search strategy
We performed a comprehensive literature search in online databases including PubMed, Embase, Web of Science, and Cochrane Library until 10 October 2017. The following search items were combined to identify qualified studies: “type 2 diabetes mellitus,” “T2DM,” “sex hormone-binding globulin,” “SHBG,” “testosterone,” and “males.” Besides, reference lists of original studies or reviews were searched manually to get more eligible literature. If more research results were needed or the information was not clear, we will contact the corresponding author for relative data. Literature fulfilling the following criteria would be enrolled in this meta-analysis: (1) English researches; (2) cross-sectional or prospective studies; (3) studies concerning the association between T2DM and testosterone.
In order to maintain the quality of the current meta-analysis, studies were excluded when (1) no clear definitions of the diagnosis of T2DM or the assessing methods of TT; (2) no clear descriptions of study design, original country, population, or the outcome assessment; (3) insufficient data for estimating a weighed mean difference (WMD) with 95% confidence interval (CI); (4) without non-T2DM control participants; (4) duplicates of the previous research. The detailed study selection process is shown in Figure S1.
Data extraction
By using a standardized data extraction form, two independent investigators reviewed the qualified researches to determine whether an individual article could be included. Uncertain data were solved by referencing original article and group discussion. The following basic characteristics and primary outcomes from each study were extracted: first author’s name, year of publication, study design, nationality, ethnicity, erectile function of the enrolled participants, length of follow-up if prospective, mean age, mean BMI, number of patients and participants in control group, serum TT, FT, and SHBG. Besides, we assessed the quality of each enrolled study by the Newcastle-Ottawa Scale (NOS) star system (range, 0–9 stars). The aforementioned data are detailed in and .
Table 1. Characteristics of studies included in the meta-analysis.
Table 2. Endpoints of variables in studies included in the meta-analysis.
Trial sequential analysis
In meta-analyses, random error can mislead findings. Due to looking on accumulating evidence when new trials emerge, the risk of random error may increase remarkably. TSA was performed to assess the random error in this meta-analysis [Citation47,Citation48]. When the cumulative Z-curve crossed by the monitoring boundaries, the result was considered convinced. In our TSA, a power of 95% (5% risk of a type II error) and 5% risk of a type I error were set. Furthermore, 5% relative risk increase was predetermined by the required information size and 95% CIs were provided. The software TSA (version 0.9; Copenhagen Trial Unit, Copenhagen, Denmark, 2011) was used in this meta-analysis.
Statistical analysis
Data were presented as mean and standard deviation (SD). If there were only standard errors (SEs) or the 95% confidence intervals of the weighted mean difference (WMD) provided, SD values will be transformed. Heterogeneity was assessed by Cochrane Q test and Higgins I2 statistic [Citation49]. If the heterogeneity was non-significant (p > 0.05 or I2<50%), a fixed-effect model would be utilized. Otherwise, a random-effect model would be applied. Pooled WMDs with corresponding 95%CIs were calculated through the inverse-variance method in fixed-effect models, or the DerSimonian–Laird method was utilized in random-effect models [Citation50]. A pooled WMD value less than 0 indicated that patients with T2DM have lower serum testosterone or SHBG levels. If the corresponding 95%CI did not include 0, the result would be considered significant. Furthermore, subgroup analyses were performed to evaluate the specific changes of testosterone and SHBG in T2DM patients from different ethnicities and erectile function. Besides, to check if there were certain low-quality studies in this meta-analysis, sensitivity analyses were performed by repeating the meta-analysis while omitting one single study each time. Meta-regression analyses were conducted to test the effect of age and BMI on WMD of TT levels. Publication bias was evaluated by a funnel plot and Begg’s test. Stata 12.0 (StataCorp LP, College Station, TX) was used to for statistics.
Results
Characteristics of the enrolled studies
Of 963 retrieved articles, 56 studies were finally enrolled in our meta-analysis (File S1), including 6856 T2DM patients and 23,572 control participants without T2DM. Among the 55 enrolled studies, nine were longitudinal researches while 47 were cross-sectional articles. Noticeably, two longitudinal studies can provide cross-sectional data and we added these two articles into the meta-analyses of cross-sectional studies. Thirty seven articles focused on Caucasian, 14 focused on Asian, four focused on African and the last one is a multi-ethnicity study. Four articles focused on T2DM patients with erectile dysfunction (ED), three focused on both ED and non-ED patients, and the other researches did not mention erectile function. Different methods were used to detect serum FT levels, including radioimmunoassay method in nine studies, equilibrium dialysis method in 13 articles, enzyme-linked immunosorbent assay in one study. Detailed aforementioned information is shown in and .
Quantitative synthesis results in cross-sectional studies
Total testosterone
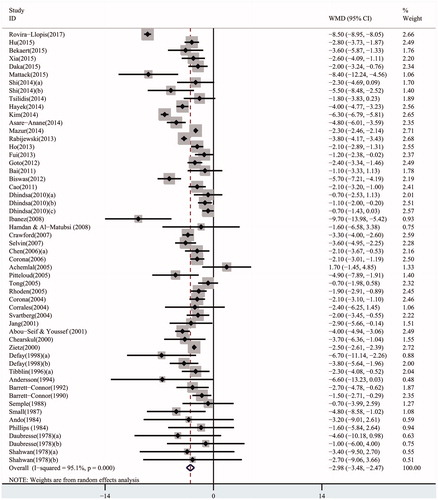
Overall, the pooled WMD of TT in cross-sectional studies was –2.98 (95% CI: –3.48 to –2.47) in a random-effect model, which demonstrated a strong association between lower serum TT levels and T2DM (). When the studies were stratified by ethnicity, the results in all ethnicities were consistent with the above analysis (Caucasian populations: pooled WMD: –2.91, 95% CI: –3.55 to –2.27; Asian populations: pooled WMD: –3.14, 95% CI: –4.30 to –1.98; African populations: pooled WMD: –2.95, 95% CI: –4.49 to –1.40) (Figure S2-1). Furthermore, in the subgroup analysis by erectile function, the results were detected significant in all groups (populations with ED: pooled WMD: –2.57, 95% CI: –3.63 to –1.52; populations without ED: pooled WMD: –5.00, 95% CI: –7.42 to –2.59; populations not assessing erectile function: pooled WMD: –2.99, 95% CI: –3.56 to –2.43) (Figure S2-2).
Free testosterone
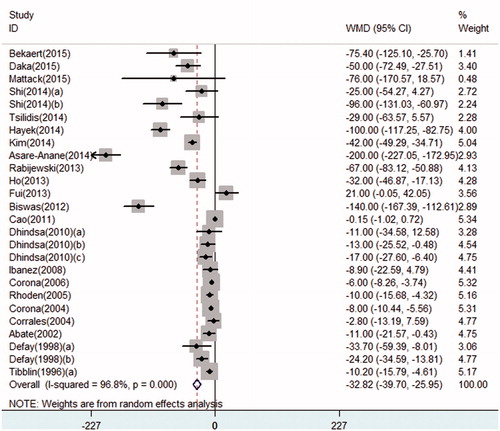
Serum FT levels significantly decreased in T2DM patients. Determined by a random-effect model, the pooled WMD of FT in cross-sectional studies was –32.82 (95% CI: –39.70 to –25.95) (). When the studies were stratified by ethnicity, FT was lower in T2DM patients in all ethnicities (Caucasian populations: pooled WMD: –21.69, 95% CI: –28.95 to –14.43; Asian populations: pooled WMD: –49.60, 95% CI: –79.24 to –19.95; African populations: pooled WMD: –75.71, 95% CI: –136.99 to –14.43) (Figure S3-1). Moreover, in the subgroup analysis by erectile function, the results were detected significant in all groups (populations with ED: pooled WMD: –20.03, 95% CI: –30.15 to –9.90; populations not assessing erectile function: pooled WMD: –36.42, 95% CI: –47.85 to –24.99), only one study provided FT data in populations without ED, and the WMD was –96.00, 95% CI: –131.03 to –60.97 (Figure S3-2). Noticeably, several methods were utilized to assess or calculate serum FT levels in enrolled studies, including radioimmunoassay method, enzyme-linked immunosorbent assay (ELISA), and a formula calculated from TT and SHBG by Vermeulen [Citation51]. Different methods might result in potential bias. Thus, we performed a subgroup analyses by detecting methods of FT. Significant results were also detected in subgroups of radioimmunoassay and formula (formula method: pooled WMD: –57.68, 95% CI: –80.81 to –34.55; radioimmunoassay method: pooled WMD: –9.40, 95% CI: –12.44 to –6.37) (Figure S3-3).
Sex hormone–binding globulin
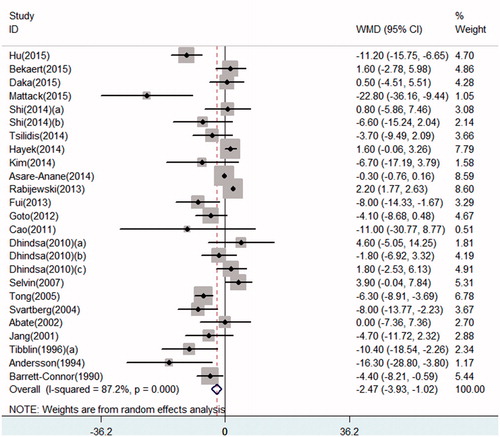
Serum SHBG levels significantly decreased in T2DM patients by a random-effect model (pooled WMD: –2.47, 95% CI: –3.93 to –1.02) (). When the studies were stratified by ethnicity, our results demonstrated a decreased in SHBG in all ethnicities (Caucasian populations: pooled WMD: –1.75, 95% CI: –4.19 to 0.69; Asian populations: pooled WMD: –5.72, 95% CI: –9.78 to –1.66). Only one study provided data concerning SHBG in African populations, the WMD in this research was –0.30 (95% CI: –0.76 to 0.16) (Figure S4). Subgroup analysis stratified by erectile function was not performed because of limited studies researching populations with ED or without ED.
Quantitative synthesis results in prospective studies
Meta-analysis results in prospective results were consistent with findings in cross-sectional studies. TT, FT, and SHBG decreased in T2DM patients (TT: pooled WMD: –2.35, 95% CI: –3.24 to –1.46 (Figure S5-1); FT: pooled WMD: –25.69, 95% CI: –83.98 to 32.05 (Figure S5-2); SHBG: pooled WMD: –0.62, 95% CI: –1.88 to 0.63 (Figure S5-3)). All the meta-analyses results are listed in .
Table 3. Meta-analyses results.
Meta-regression results
Testosterone levels are associated with BMI and age according to various published studies. However, in this meta-analysis, meta-regression in cross-sectional studies showed that WMD in TT was not associated with age (p = 0.256) or BMI (p = 0.491) (Figure S6A, B).
TSA results
For the first time, TSA was conducted to verify the pooled results in our meta-analysis. The cumulative Z-curve has crossed the monitoring boundaries. Meanwhile, the number of the T2DM patients and non-T2DM participants has achieved the required information size (Figure S7A, B). TSA results showed that our results were based on sufficient evidence.
Sensitivity analysis
In the current meta-analysis, no alterations in pooled WMDs was found after any study was excluded (Figure S8), demonstrating that no individual research can significantly affect the pooled WMDs. Accordingly, sensitivity analysis made our results more reliable.
Publication bias
Evidence of obvious asymmetrical was not found in the funnel plot (Figure S9). Furthermore, Begg’s test cannot found significant publication bias in our meta-analysis (TT in cross-sectional studies: p = 0.070; FT in cross-sectional studies: p = 0.415; SHBG in cross-sectional studies: p = 0.455; TT in prospective studies: p = 0.677).
Discussion
The incidence rate of hypogonadism in patients with T2DM is higher than general population. Testosterone supplement therapy is effective for hypogonadal patients with T2DM and there are a number of potential clinical benefits, including improvements in glycemic control, insulin resistance, muscle strength, increased lean mass, libido, and erectile function in hypogonadal patients with T2DM [Citation52]. Inversely, androgen deprivation therapy may impair glycemic control and increase cardiovascular biochemical risk factors after androgen deprivation in men with prostate cancer insulin-dependent diabetes [Citation53]. The higher morbidity and the effectiveness of testosterone treatment have increased attention of urology practitioners. According to the guidelines of European Association of Urology in 2017 and American Diabetes Association in 2018, measurement of testosterone in men with type 2 diabetes and testosterone supplement therapy for symptomatic hypogonadal men who are not considering parenthood are recommended. These symptoms were not restricted to erectile dysfunction. Symptoms such as depression, loss of libido, erectile dysfunction, and other physical and emotional abnormalities can be treated by testosterone therapy.
Consistent with most articles’ results [Citation20,Citation21], the current meta-analysis demonstrated significant decrease in serum TT, FT, and SHBG levels in adult males with T2DM by pooling the data of all cross-sectional studies. Noticeably, since free testosterone appears higher as a result of decreased SHBG, SHBG declines in diabetes decrease the clinical value of free testosterone. Specific role of free testosterone in these patients is required to be clarified. In longitudinal studies, a significant decrease in TT was found. However, the decrease in FT and SHBG were non-significant in prospective studies, which might result from limited longitudinal articles researching FT and SHBG. More studies were required to get a more comprehensive result.
In subgroup analyses stratified by ethnicity, male patients with T2DM demonstrated decrease of TT, FT, and SHBG in all subgroups. Whatever TT, FT, or SHBG, WMD of the Caucasian populations were the highest, African populations were the second and the Asian populations were the lowest. Ethnicities might influence testosterone changes in T2DM patients according to our meta-analysis.
Hypogonadism is directly associated with ED according to various studies, especially in patients with T2DM [Citation54,Citation55], which might be associated with abnormities concerning mean weight, waist circumference, triglycerides, total cholesterol, HbA1c, fasting glucose, and AMS scores in patients with late-onset hypogonadism [Citation56,Citation57]. On the other side, testosterone supplement therapy can significantly improve erectile function and other sexual parameters as measured by IIEF in hypogonadal men [Citation58]. However, studies focusing on the sexual function of hypogonadal patients with T2DM are limited and only seven studies reported erectile function in this meta-analysis. Noticeably, ED was self-reported in two studies. According to a previously published study [Citation59], the prevalence of ED might be underestimated if assessed by IIEF. When the subgroups were stratified by erectile function, male patients with T2DM demonstrated decrease of TT and FT in all subgroups, while WMD of TT and FT in participants without ED are relatively lower than those participants with ED. More studies are required for further understanding of the effect of testosterone in erectile function in T2DM patients.
Older age and higher BMI were associated with lower testosterone according to various published studies. Meanwhile, testosterone supplement therapy is able to increase muscle mass in elderly men [Citation60]. Although BMI and age can directly influence serum TT level, meta-regression in cross-sectional studies demonstrated a that WMD in T2DM patients cannot be affected by these factors. The diagnosis of late-onset hypogonadism (LOH) is based on a combination of decreased testosterone and a series of symptoms such as depression, loss of libido, erectile dysfunction, and other physical and emotional abnormalities. Although testosterone level naturally decreases with aging and obesity, some aged or obese men present with the symptoms of LOH, but with normal testosterone levels, and some men with low testosterone levels have no symptoms. Moreover, low testosterone in the absence of any symptoms does not clearly need to be treated. From this point, LOH is a function condition rather than an aged-associated or BMI-associated disease, which is consistent with our meta-analysis results.
TSA is a tool for evaluating the random errors in meta-analysis. In our study, Z-curves have crossed the monitoring boundaries and the sample size of T2DM patients and control participants has achieved the required information size. Moreover, we compared our meta-analysis of prospective studies with a previous published meta-analysis by Corona et al, which included five prospective studies. As shown in Figure S7B, the total size of participants was less than the estimated information size in the previous five prospective studies. There might be some potential random errors due to limited sample size. After adding another four prospective studies, the sample size has achieved the estimated value while the cumulative Z-curve crossed the monitoring boundaries, which indicated that our results were sufficient.
There are several advantages in our meta-analysis: (1) compared with the meta-analysis in 2010, the sample size almost doubled, which made our results more convinced. (2) Begg’s tests and sensitivity analyses results demonstrated no publication bias or low-quality studies. (3) TSA was performed for the first time in our study and Z-curves have crossed the monitoring boundaries in both cross-sectional and prospective studies.
Despite the overall sufficient results, there are some limitations in our meta-analysis: (1) the sample sizes in several subgroups are limited. For instance, only one study provided data concerning SHBG in African populations and only another study provided FT data in populations without ED. More studies are required for a more comprehensive understanding of testosterone in T2DM in these subgroups. (2) Although TSA results showed that TT has a firm decrease in T2DM patients, more studies of high quality are required to offer more detailed individual data.
Conclusion
Our results indicate that male patients with T2DM have a lower serum TT, FT, and SHBG levels.
File_S1.docx
Download MS Word (19.2 KB)Supplement_figures.docx
Download MS Word (11.1 MB)Disclosure statement
The authors declare none conflicts of interest in this study.
Additional information
Funding
References
- Jiang X, Ma H, Wang Y, et al. Early life factors and type 2 diabetes mellitus. J Diabetes Res. 2013;2013:485082.
- Strachan MW, Reynolds RM, Frier BM. The role of metabolic derangements and glucocorticoid excess in the aetiology of cognitive impairment in type 2 diabetes. Implications for future therapeutic strategies. Diabetes Obes Metab. 2009;11:407–414.
- Asfaha S, Padwal R. Antihypertensive drugs and incidence of type 2 diabetes: evidence and implications for clinical practice. Curr Hypertens Rep. 2005;7:314–322.
- Elnaem MH, Mohamed MHN, Huri HZ, et al. Statin therapy prescribing for patients with type 2 diabetes mellitus: a review of current evidence and challenges. J Pharm Bioallied Sci. 2017;9:80–87.
- Canguven O, Talib RA, El Ansari W, et al. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017;20:9–16.
- Basat S, Sivritepe R, Ortaboz D, et al. The relationship between vitamin D level and erectile dysfunction in patients with type 2 diabetes mellitus. Aging Male. 2018;21:111–115.
- Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2018;1–12. DOI:10.1080/13685538.2018.1482487
- Deng C, Zhang Z, Li H, et al. Analysis of cardiovascular risk factors associated with serum testosterone levels according to the US 2011–2012 National Health and Nutrition Examination Survey. Aging Male. 2018;1–8. DOI:10.1080/13685538.2018.1479387
- Rabijewski M, Papierska L, Kuczerowski R, et al. Hormonal determinants of erectile dysfunction and lower urinary tract symptoms in middle-aged and elderly men with prediabetes. Aging Male. 2015;18:256–264.
- Khripun I, Vorobyev S, Belousov I, et al. Influence of testosterone substitution on glycemic control and endothelial markers in men with newly diagnosed functional hypogonadism and type 2 diabetes mellitus: a randomized controlled trial. Aging Male. 2018;1–9. DOI:10.1080/13685538.2018.1506918
- Morgunov LY, Denisova IA, Rozhkova TI, et al. Hypogonadism and its treatment following ischaemic stroke in men with type 2 diabetes mellitus. Aging Male. 2018;1–10. DOI:10.1080/13685538.2018.1487932
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a Controlled Registry Study. J Urol. 2018;199:257–265.
- Groti K, Zuran I, Antonic B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus – a series of case reports. Aging Male. 2015;18:164–168.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19:64–69.
- Hatami H, Parizadeh D, Bidhendi Yarandi R, et al. Endogenous testosterone does not improve prediction of incident cardiovascular disease in a community-based cohort of adult men: results from the Tehran Lipid and Glucose Study. Aging Male. 2018;1–8. DOI:10.1080/13685538.2018.1466876
- Livingston M, Kalansooriya A, Hartland AJ, et al. Serum testosterone levels in male hypogonadism: why and when to check – a review. Int J Clin Pract. 2017;71. DOI:10.1111/ijcp.12995
- Lu Y, Li J, Cheng X, et al. Testosterone level in aging male with different glucose tolerance state and its association with osteocalcin. Aging Male. 2018;1–6. DOI:10.1080/13685538.2018.1481940
- Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299.
- Bai J, Liu Y, Niu GF, et al. Relationship between adiponectin and testosterone in patients with type 2 diabetes. Biochem Med (Zagreb). 2011;21:65–70.
- Achemlal L, Tellal S, Rkiouak F, et al. Bone metabolism in male patients with type 2 diabetes. Clin Rheumatol. 2005;24:493–496.
- Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34:528–540.
- Atlantis E, Fahey P, Martin S, et al. Predictive value of serum testosterone for type 2 diabetes risk assessment in men. BMC Endocr Disord. 2016;16:26.
- Salminen M, Vahlberg T, Raiha I, et al. Sex hormones and the risk of type 2 diabetes mellitus: a 9-year follow up among elderly men in Finland. Geriatr Gerontol Int. 2015;15:559–564.
- Li JJ, Wittert GA, Vincent A, et al. Muscle grip strength predicts incident type 2 diabetes: population-based cohort study. Metab Clin Exp. 2016;65:883–892.
- Svartberg J, Schirmer H, Wilsgaard T, et al. Single-nucleotide polymorphism, rs1799941 in the Sex Hormone-Binding Globulin (SHBG) gene, related to both serum testosterone and SHBG levels and the risk of myocardial infarction, type 2 diabetes, cancer and mortality in men: the Tromsø Study. Andrology. 2014;2:212–218.
- Bekaert M, Van Nieuwenhove Y, Calders P, et al. Determinants of testosterone levels in human male obesity. Endocrine. 2015;50:202–211.
- Daka B, Langer RD, Larsson CA, et al. Low concentrations of serum testosterone predict acute myocardial infarction in men with type 2 diabetes mellitus. BMC Endocr Disord. 2015;15:35.
- Hu J, Zhang A, Yang S, et al. Combined effects of sex hormone-binding globulin and sex hormones on risk of incident type 2 diabetes. J Diabetes. 2016;8:508–515.
- Mattack N, Devi R, Kutum T, et al. The evaluation of serum levels of testosterone in type 2 diabetic men and its relation with lipid profile. J Clin Diagn Res. 2015;9:*BC04–BC07.
- Rovira-Llopis S, Banuls C, de MAM, et al. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic Biol Med. 2017;108:155–162.
- Shi MD, Chao JK, Ma MC, et al. The connection between type 2 diabetes and erectile dysfunction in Taiwanese aboriginal males. Int J Impot Res. 2014;26:235–240.
- Xia JW, Tan SJ, Zhang XL, et al. Correlation of serum testosterone with insulin resistance in elderly male type 2 diabetes mellitus patients with osteoporosis. J Diabetes Invest. 2015;6:548–552.
- Al Hayek AA, Khawaja NM, Khader YS, et al. The prevalence of hypogonadism among diabetic and non-diabetic men in Jordan. J Diabetes Complicat. 2014;28:135–140.
- Asare-Anane H, Ofori E, Agyemang Y, et al. Obesity and testosterone levels in Ghanaian men with type 2 diabetes. Clin Diabetes. 2014;32:61–65.
- Biswas M, Hampton D, Newcombe RG, et al. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin Endocrinol (Oxf). 2012;76:665–673.
- Cao J, Li J, Hao W, et al. Correlation of sex hormone and androgen receptor with diabetes mellitus in elderly men. Aging Male. 2011;14:162–167.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–1192.
- Goto A, Morita A, Goto M, et al. Associations of sex hormone-binding globulin and testosterone with diabetes among men and women (the Saku Diabetes study): a case control study. Cardiovasc Diabetol. 2012;11:130.
- Ho CH, Yu HJ, Wang CY, et al. Prediabetes is associated with an increased risk of testosterone deficiency, independent of obesity and metabolic syndrome. PLoS One. 2013;8:e74173.
- Kim KS, Kang SH, Kim MJ, et al. Low serum testosterone concentrations in hospitalized men with poorly controlled type 2 diabetes. Endocrinol Metab (Seoul). 2014;29:574–578.
- Mazur A, Westerman R, Werdecker A, et al. Testosterone and type 2 diabetes in men. Aging Male. 2014;17:18–24.
- Ng Tang Fui M, Hoermann R, Cheung AS, et al. Obesity and age as dominant correlates of low testosterone in men irrespective of diabetes status. Andrology. 2013;1:906–912.
- Rabijewski M, Papierska L, Zgliczyński W, et al. The incidence of hypogonadotropic hypogonadism in type 2 diabetic men in Polish population. Biomed Res Int. 2013;2013:767496.
- Tsilidis KK, Allen NE, Appleby PN, et al. Diabetes mellitus and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;136:372–381.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269.
- Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75.
- Zhang J, Xiao L, Qin Z, et al. Association between germline homeobox B13 (HOXB13) G84E allele and prostate cancer susceptibility: a meta-analysis and trial sequential analysis. Oncotarget. 2016;7:67101–67110.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672.
- Haider A, Yassin A, Doros G, et al. Effects of long-term testosterone therapy on patients with “diabesity”: results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:683515.
- Haidar A, Yassin A, Saad F, et al. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196.
- Li HJ. More attention should be paid to the treatment of male infertility with drugs-testosterone: to use it or not? Asian J Androl. 2014;16:270–273.
- Hackett G, Cole N, Saghir A, et al. Testosterone replacement therapy: improved sexual desire and erectile function in men with type 2 diabetes following a 30-week randomized placebo-controlled study. Andrology. 2017;5:905–913.
- Panach-Navarrete J, Martinez-Jabaloyas JM. The influence of comorbidities on the aging males’ symptoms scale in patients with erectile dysfunction. Aging Male. 2017;20:146–152.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18:186–194.
- Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of results of testosterone therapy on sexual function based on International Index of Erectile Function Scores. Eur Urol. 2017;72:1000–1011.
- Ahn TY, Park JK, Lee SW, et al. Prevalence and risk factors for erectile dysfunction in Korean men: results of an epidemiological study. J Sex Med. 2007;4:1269–1276.
- Neto WK, Gama EF, Rocha LY, et al. Effects of testosterone on lean mass gain in elderly men: systematic review with meta-analysis of controlled and randomized studies. Age (Dordr). 2015;37:9742.