Abstract
Introduction
To investigate possible pre-treatment factors related to the therapeutic effect of tadalafil on bladder outlet obstruction (BOO).
Materials and methods
Eighty untreated outpatients with lower urinary tract symptoms (LUTS) due to BOO received 5 mg tadalafil daily for 12 months. Subjective symptoms and objective findings were evaluated before and 12 months after treatment. At 12 months, the patients were divided into two groups according to an improvement grade in BOO index (BOOI). Patient characteristics including age, serum total testosterone level (TT), PSA, and prostate volume, and subjective and objective parameters on LUTS were set as candidates of pre-treatment factors, and the parameters that influenced the improvement of BOO were statistically analysed.
Results
A total of 69 patients with mean age of 69.8 years and mean prostate volume of 48.8 mL were included. Subjective symptoms and BOOI were significantly ameliorated after 12 months. In terms of an improvement of BOOI, 30 patients (43.5%) showed insignificant improvement in BOO, whereas 39 patients (56.5%) exhibited excellent improvement. Comparison of pre-treatment factors between the groups showed that TT was the only independent predictor related to the improvement in BOO. The improvement of BOO was significantly better in patients with higher TT.
Conclusions
Pre-treatment TT was considered to be a useful predictor of therapeutic effects of tadalafil for BOO.
Introduction
Curative drugs for benign prostatic hyperplasia (BPH) have been reported to improve lower urinary tract symptoms (LUTS), and relieving bladder outlet obstruction (BOO) is considered the main mechanism [Citation1–3]. However, in real clinical practice, some patients fail to obtain an improvement in subjective symptoms or BOO. Thus, identifying patients who will not respond to pharmacotherapy ahead of drug administration is important. With regard to predictive factors for therapeutic effects of α1-adrenoceptor antagonists (α1-blockers) which are widely used as first-choice drug for LUTS suggestive of BPH (LUTS/BPH), prostate volume, and severity of pre-treatment subjective symptoms reportedly affect their therapeutic efficacy [Citation4,Citation5]. Recently, we have reported that intravesical prostatic protrusion (IPP) is a useful predictor of the therapeutic effects of silodosin for subjective symptoms and BOO [Citation6].
A phosphodiesterase 5 (PDE5) inhibitor is also recommended as the first-line drug for LUTS/BPH by BPH guidelines in several countries [Citation1–3]. Tadalafil, the only PDE5 inhibitor approved for patients with LUTS/BPH, significantly improves LUTS [Citation7,Citation8]. We reported that tadalafil significantly improved not only subjective symptoms but also objective storage and voiding function evaluated by urodynamic studies in patients with LUTS/BPH during a 1-year treatment [Citation9]. However, some studies reported that tadalafil failed to improve objective parameters for voiding functions such as BOO and urinary flow rate [Citation10–12]. Whether the effects of tadalafil on objective findings, such as BOO, are not observed or marginal in some patients is not clearly elucidated. Few studies have focussed on which patients have a weak efficacy in improvement of objective symptoms in the treatment of tadalafil. Based on these backgrounds, we conducted the present study, aiming to (i) investigate whether tadalafil improves BOO in patients with benign prostatic obstruction (BPO), and (ii) clarify the possible pre-treatment factors related to the therapeutic effects on BOO in the treatment of tadalafil.
Materials and methods
This study was a single-centre, open label, prospective, and conducted in accordance with the ethical principles of the Declaration of Helsinki. The ethics committee of the Nagoya University Graduate School of Medicine approved this study protocol (IRB approval number: 2015–0106). All participants provided written informed consent before enrolment.
Among the treatment-naive men with LUTS/BPH, the patients with BPO in addition to LUTS were analysed in this study. The inclusion criteria were as follows: total international prostate symptom score (IPSS) ≥ 8; IPSS-quality of life (QOL) score ≥ 3; prostate volume ≥ 25 ml as determined by transabdominal ultrasonography; age ≥ 50 years; and bladder outlet obstruction index (BOOI) >40 as determined by pressure flow study (PFS). BOOI was calculated according to the following formula: detrusor pressure at Qmax (PdetQmax) – 2 × maximum urinary flow rate (Qmax), according to the International Continence Society nomogram. Patients were excluded if they had received oral treatment with α1-blockers, anticholinergic agents, 5α-reductase inhibitors, antidepressants, anti-anxiety agents, sex hormone agents, nitroglycerine, amyl nitrite, or isosorbide dinitrate. Medical conditions for exclusion were neurogenic bladder dysfunction, bladder calculi, active urinary tract infection, severe cardiac disease, renal dysfunction (serum creatinine level ≥ 2 mg/dL), and/or hepatic dysfunction (aspartate and alanine aminotransferase concentrations more than twice the normal values). To ensure that only cancer-free patients were included, prostate biopsy was performed in all patients who had prostate-specific antigen (PSA) levels > 4 ng/mL.
Patients who satisfied all the inclusion and exclusion criteria received tadalafil (5 mg/day) for 12 months. To monitor subjective symptom severity and QOL in the patients, IPSS, IPSS-QOL, and overactive bladder symptom scores (OABSS) were measured before and 12 months after treatment. The same schedule was implemented to evaluate voiding functions in subjected patients by urodynamic studies (UDS) including uroflowmetry (UFM) and PFS. Qmax, post-void residual urine volume (PVR), PdetQmax, and BOOI were determined as parameters of voiding function. UDS was performed according to the standard methods defined by the International Continence Society [Citation13,Citation14]. We evaluated the effect of tadalafil on BOO and subjective symptoms in patients with BPO.
The target enrolment number was calculated based on previous studies and a clinical relevant change in BOOI of 16 was adopted [Citation15]. The expected mean change (standard deviation) for the change of BOOI from baseline was assumed to be 10 (15). The sample size required to determine this change was 68 when the two-sided alpha level and power were assumed to be 5% and 90%, respectively. Finally, a total of 80 cases were calculated as the required number of cases, considering a dropout rate of 15%.
We also divided the patients into two groups based on the grade of improvement in BOOI. Patients with a BOOI improvement of 25% or more were classified as good responders (BOO-GR) and those with improvement of less than 25% were classified as poor responders (BOO-PR), because the mean improvement rate of BOOI which resulted from 12 weeks administration of tadalafil was 23.2% in our previous study [Citation15]. We compared the backgrounds of the two groups and evaluated the pre-treatment factors related to therapeutic effects on BOO improvement.
To assess the impact of pre-treatment factors on therapeutic effects, we set the following parameters: age, serum PSA level, serum total testosterone (TT) level, prostate volume, IPSS, IPSS voiding score, IPSS storage score, OABSS, Qmax, and PVR (which was obtained on UFM), and IPP. IPP was measured as previously reported [Citation16]. IPP and prostate volume were measured by one laboratory technician who was not involved in this study. Serum TT level (normal range in our lab was from 2.0 to 9.2 ng/mL) was measured between 9:00 and 10:00 am to minimise the confounding effects of diurnal variation in all patients.
All statistical values were presented as mean ± standard deviation. The Wilcoxon signed-rank test was utilised to evaluate the changes in subjective symptoms and objective findings. Mann–Whitney U test and multiple linear regression analysis were performed to determine the significant pre-treatment factors for therapeutic effects of tadalafil. All tests were two-sided, and p < .05 was considered statistically significant. Statistical analysis was performed using SPSS software (IBM, Armonk, NY).
Results
Out of the 80 patients who were diagnosed to have not only LUTS but also BOO at pre-treatment UDS evaluation, 4 (5.0%) discontinued treatment due to adverse reactions including headache (n = 2), diarrhoea (n = 1), and dizziness (n = 1). In addition, two patients developed urinary retention during the study period, and five were not subjected to PFS after treatment initiation. Therefore, the analyses included 69 patients with mean age of 69.8 years (range 51–82) and mean prostate volume of 48.8 ml (range 29–108 ml). All study participants were Japanese.
The changes in subjective and objective symptoms are summarised in . Subjective parameters on LUTS, including IPSS, OABSS, and IPSS-QOL, significantly improved at one year after tadalafil administration. Regarding voiding function, the parameters on BOO, such as Qmax and PdetQmax, significantly improved after treatment. Mean BOOI also significantly improved from 71.3 to 50.2 (−21.1; −29.6%) one year after administration. PVR significantly decreased by 24 ml after 12 months compared with the baseline value.
Table 1. The change of subjective and objective parameters before and after tadalafil administration in patients with BPO.
Thirty patients (43.5%) had a decline of less than 25% in BOOI and were classified as poor responders (BOO-PR), while 39 patients (56.5%) demonstrated a BOOI improvement of more than 25% and were classified as good responders. Mean BOOI in the BOO-GR significantly improved from 71.8 to 39.3 (p < .001), and a mean improvement rate was 44.3%. Mean BOOI in the BOO-PR changed from 70.6 to 64.3 (p = .25), which was not a significant improvement. The number of patients demonstrating BOO on PFS decreased from 39 to 15 patients (61.5%) in the BOO-GR and 31 to 29 (6.5%) in the BOO-PR one year after treatment.
With regard to a univariate comparison of pre-treatment factors between the two groups according to the improvement of BOO, although no difference was found in age, prostate volume, serum PSA level, IPSS, OABSS, Qmax, and degree of IPP between the two groups, the pre-treatment serum TT level was the only significant factor related to therapeutic effect on BOO ().
Table 2. The comparison of pre-treatment factor between the two groups according to the improvement of BOOI.
During analysis of pre-treatment factors correlated with improvement in BOOI, a significant positive correlation was observed between TT at baseline and improvement in BOOI (r = 0.33, p = .003). Additionally, multiple linear regression analysis showed that TT was the only significant predictive factor of BOO improvement (). With regard to the improvement of BOO, a receiver-operating curve (ROC) analysis identified 5.0 ng/mL of serum testosterone level as the optimal cut-off value; this value yielded a sensitivity of 75% and a specificity of 64% to predict BOO improvement ().
Figure 1. ROC curve of serum total testosterone level regarding the improvement of BOO. ROC analysis identified 5.0 ng/mL of serum total testosterone level as the optimal cut-off value; this value yielded a sensitivity of 75% and a specificity of 64%.
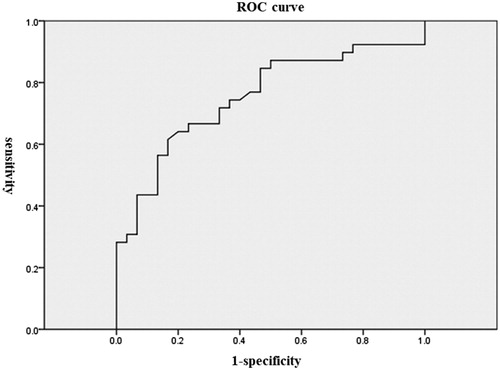
Table 3. Results of correlation analysis and stepwise multiple regression analysis to select factors related to therapeutic effects in BOO.
Discussion
The use of α1-blocker or PDE5 inhibitor is recommended as the first choice drug for patients with LUTS/BPH according to current BPH guidelines of several countries [Citation1–3]. However, how to use these drugs separately, or which patients tend to respond to these drugs has not been clearly elucidated. Oelke et al. [Citation17] investigated the efficacy of tadalafil 5 mg on subjective symptoms in the treatment of LUTS/BPH according to patient age. They concluded that the patients aged <75 years had a significantly greater improvement in IPSS, IPSS storage sub-score, IPSS voiding sub-score and IPSS-QOL index, compared with those aged ≥75 years. In addition, Vlachopoulos et al. [Citation18] reported that the efficacy of tadalafil on LUTS was significantly poorer in patients with multiple drug treatment for arterial hypertension than those without corresponding conditions. However, no study has evaluated which patient background has influenced on the ameliorating effect of tadalafil, in particular, focussing on the changes in objective symptoms, such as BOO. To our knowledge, this is the first study to investigate the factors related to the therapeutic effects of tadalafil on BOO in patients with LUTS/BPO. Our finding that serum TT level had a significant influence on the effect of tadalafil on BOO is a useful information for clinicians in selecting a therapeutic strategy for patients with LUTS/BPH. We previously investigated possible factors related to therapeutic failure in the treatment by silodosin and reported that IPP could be a predicting factor for the therapeutic effects of silodosin in males with LUTS/BPH [Citation6]. In the present study, while the serum TT level was a significant factor to predict the effect of tadalafil, IPP was not. This finding may be contributory to consideration of a therapeutic strategy for patients with LUTS/BPH in choice of α1-blocker or PDE5 inhibitor.
Dmochowski et al. [Citation10] reported no significant difference between tadalafil and placebo treatment in terms of mean change in any urodynamic parameter, such as BOOI and Qmax, for male patients with LUTS. They reported that the mean change of BOOI from baseline to the end point was −2.8 in patients treated with tadalafil. However, in their study, the mean BOOI at baseline was 35.6, and approximately 70% of all patients had no BOO (BOOI ≤ 40). Pathophysiological change in male patients with LUTS/BPH was thought to include not only BOO but also detrusor underactivity (DU). Recently, Osman et al. [Citation19] reported that DU was present in 9–48% of men undergoing urodynamic evaluation for non-neurogenic LUTS. In the present study, because we intended to evaluate whether tadalafil improved BOO in patients with LUTS suggestive of BPO and to investigate factors related to the effect of tadalafil on purely BOO, we targeted only patients with BOO. Consequently, we showed that treatment with tadalafil for 12 months significantly relieved voiding function such as Qmax and BOOI, along with LUTS, in patients diagnosed with LUTS and BPO. Moreover, in the evaluation of factors related to the improvement of BOO by tadalafil treatment, no difference in pre-treatment urodynamic parameters such as BOOI, Qmax, and PVR, was found between the BOO-GR and BOO-PR groups. The evaluation with more accuracy can be conducted by precluding any possibility of patients whose BOOI was 40 or less.
Although testosterone replacement therapy was reported to improve LUTS in patients with hypogonadism and BPH [Citation20,Citation21], it was interesting that that serum TT level was a significant predicting factor for therapeutic effects of tadalafil on BOO. The detailed mechanism remains incompletely understood, but the plausible hypotheses for this finding include the following. First, Zhang Xi et al. [Citation22] indicated that surgical castration induced a significant reduction of PDE5 gene using the rat corpus cavernosum and that tadalafil treatment was ineffective in ameliorating the electro-stimulation response in castrated rats. They concluded that testosterone positively regulated PDE5 expression and in vivo responsiveness to tadalafil in the rat corpus cavernosum. Additionally, some articles reported that the expression of PDE5 appeared androgen-dependent in human and testosterone deficiency predicted a poor response to sildenafil, one of the PDE5 inhibitors, in the improvement of erectile function [Citation23,Citation24]. Based on these backgrounds, in patients with lower TT level at baseline, the PDE5 expression in the prostate and bladder outlet might be estimated to decrease. Consequently, the ameliorating effect of tadalafil on voiding function may be attenuated. Second, low serum testosterone level was reported to be an independent determinant of endothelial dysfunction in men [Citation25,Citation26]. In addition, Hak et al. [Citation27] found an independent inverse association between the testosterone level and aortic atherosclerosis. They concluded that low serum testosterone level increased the risk of atherosclerosis in elderly men. Based on these findings, the degradation of vascular endothelial function induced by low serum testosterone could cause the loss of smooth muscle and the decrease of blood flow in the bladder and prostate. Consequently, the effect of tadalafil on BOO was thought to be weak in patients with low serum TT level. In our study, the mean serum total testosterone level in the BOO-PR group and the BOO-GR group were within normal range. However, there was a significant difference in the improvement of BOO between the two groups. Our data suggest that the serum TT level may have a significant influence on the effect of tadalafil on BOO, even within a normal range. Further investigation should be necessary to elucidate the relationship between testosterone level and effect of tadalafil on BOO.
The present study has several limitations. First, the number of cases might be insufficient to perform multivariate analysis. Although we could evaluate the factor related to the therapeutic effects of tadalafil in a univariate analysis, further studies with more subjects will be required in the future to confirm the present results. Second, some parameters, such as medical complications including cardiovascular diseases and diabetes mellitus, were not evaluated. Higher age or coexisting cardiovascular diseases such as hypertension, which have been reported to be significant factors related to the effect on LUTS [Citation17,Citation18], would also cause a decrease of vascular endothelial function and pelvic blood flow, similar to low serum TT. Because, the interconnectedness between these factors is clearly unknown, further studies are warranted to identify the most reliable or correlated factor.
Conclusions
Tadalafil was effective in the improvement of BOO for patients with LUTS due to BPO. Especially, a higher ameliorating effect on improving BOO was observed in patients with higher serum TT level (cut-off value of 5.0 ng/mL). Pre-treatment serum TT level was considered to be a useful predictor of therapeutic effects of tadalafil on BOO.
Acknowledgements
We thank all patients for participating and all trial investigators for their contribution to data acquisition and patient care.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Homma Y, Gotoh M, Kawauchi A, et al. Clinical guidelines for male lower urinary tract symptoms and benign prostatic hyperplasia. Int J Urol. 2017;24:716–729.
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67:1099–1109.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803.
- Mochtar CA, Kiemeney LA, Laguna MP, et al. Prognostic role of prostate-specific antigen and prostate volume for the risk of invasive therapy in patients with benign prostatic hyperplasia initially managed with alpha1-blockers and watchful waiting. Urology. 2005;65:300–305.
- Masumori N, Hashimoto J, Itoh N, et al. Short-term efficacy and long-term compliance/treatment failure of the alpha1 blocker naftopidil for patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Scand J Urol Nephrol. 2007;41:422–429.
- Matsukawa Y, Ishida S, Majima T, et al. Intravesical prostatic protrusion can predict therapeutic response to silodosin in male patients with lower urinary tract symptoms. Int J Urol. 2017;24:454–459.
- Gacci M, Andersson KE, Chapple C, et al. Latest evidence on the use of phosphodiesterase type 5 Inhibitors for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2016;70:124–133.
- Chapple CR, Roehrborn CG, McVary K, et al. Effect of tadalafil on male lower urinary tract symptoms: an integrated analysis of storage and voiding international prostate symptom subscores from four randomised controlled trials. Eur Urol. 2015;67:114–122.
- Matsukawa Y, Takai S, Majima T, et al. Objective impacts of tadalafil on storage and voiding function in male patients with benign prostatic hyperplasia: 1-year outcomes from a prospective urodynamic study. World J Urol. 2018. doi:10.1007/s00345-018-2453-x
- Dmochowski R, Roehrborn C, Klise S, et al. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol. 2010;183:1092–1097.
- Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201.
- Kim SC, Park JK, Kim SW, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in korean men with benign prostatic hyperplasia: results from a placebo-controlled pilot study using tamsulosin as an active control. Low Urin Tract Symptoms. 2011;3:86–93.
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178.
- Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274.
- Matsukawa Y, Majima T, Matsuo K, et al. Effects of tadalafil on storage and voiding function in patients with male lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a urodynamic-based study. Int J Urol. 2018;25:246–250.
- Matsukawa Y, Kato M, Funahashi Y, et al. What are the predicting factors for the therapeutic effects of dutasteride in male patients with lower urinary tract symptoms? Investigation using a urodynamic study. Neurourol Urodyn. 2017;36:1809–1815.
- Oelke M, Wagg A, Takita Y, et al. Efficacy and safety of tadalafil 5 mg once daily in the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia in men aged ≥75 years: integrated analyses of pooled data from multinational, randomized, placebo-controlled clinical studies. BJU Int. 2017;119:793–803.
- Vlachopoulos C, Oelke M, Maggi M, et al. Impact of cardiovascular risk factors and related comorbid conditions and medical therapy reported at baseline on the treatment response to tadalafil 5 mg once-daily in men with lower urinary tract symptoms associated with benign prostatic hyperplasia: an integrated analysis of four randomised, double-blind, placebo-controlled. Int J Clin Pract. 2015;69:1496–1507.
- Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. Eur Urol. 2014;65:389–398.
- Shigehara K, Sugimoto K, Konaka H, et al. Androgen replacement therapy contributes to improving lower urinary tract symptoms in patients with hypogonadism and benign prostate hypertrophy: a randomised controlled study. Aging Male. 2011;14:53–58.
- Amano T, Imao T, Takemae K, et al. Testosterone replacement therapy by testosterone ointment relieves lower urinary tract symptoms in late onset hypogonadism patients. Aging Male. 2010;13:242–246.
- Zhang XH, Morelli A, Luconi M, et al. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47:409–416.
- Hwang TI, Chen HE, Tsai TF, et al. Combined use of androgen and sildenafil for hypogonadal patients unresponsive to sildenafil alone. Int J Impot Res. 2006;18:400–404.
- Morelli A, Filippi S, Mancina R, et al. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–2263.
- Filippi S, Vignozzi L, Morelli A, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–3288.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res. 2007;30:1029–1034.
- Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639.
