Abstract
Aim
Obstructive sleep apnea syndrome (OSAS) is a chronic and incapacitating disease that often requires lifelong care. This study aimed to evaluate the thiol/disulfide homeostasis in patients with OSAS, to compare the thiol/disulfide levels with the control group and to investigate their relationship with the severity of the disease.
Material and methods
Patients who were admitted to the department of chest diseases, and diagnosed with OSAS using polysomnographic analysis (n = 186) and 144 patients who underwent polysomnography due to some reasons but ruled out of having OSAS were included in the study. Serum total thiol (TT), native thiol (SH), and disulfide thiol (SS) levels were measured from the participants; SS/SH, SS/TT, and SH/TT percent ratios were calculated and compared between the patient and control groups.
Results
The mean (±SD) age of the patients and control participants was 52.0 ± 11.5 years and 44.9 ± 13.2 years, respectively. Compared to the control group, patients with OSAS had significantly lower SH (239.3 ± 56.3 μmol/L vs. 258.6 ± 65.3μmol/L, t = 2.70, p =.007) and TT levels (273.2 ± 60.1 μmol/L vs. 292.9 ± 67.5μmol/L, t = 2.64, p=.010). Age (OR = 1.04), serum albumin (OR = 12.67), ischemia-modified albumin (IMA) (OR = 0.12), SH (OR = 0.81), and TT (OR = 1.17) were independent predictors of OSAS.
Conclusions
These results support the idea that decreased ST and TT levels are related to increased oxidative stress. On the other hand, impaired thiol balance may play a significant role in the pathogenesis of OSAS.
Introduction
Background/rationale
Obstructive sleep apnea syndrome (OSAS) is a chronic and devastating disease that may lead to a lifelong burden [Citation1]. OSAS has typically recurrent episodes of upper respiratory tract collapse during sleep [Citation2]. The episodes of respiratory collapse lead to repeated oxyhemoglobin desaturations and awakening from sleep. OSAS is an overwhelming disease because of poor neurocognitive performance due to hypoxia lasting for years and the many other adverse medical consequences. OSAS is linked to many diseases such as increased cardiovascular mortality, hypertension, insulin resistance, type 2 diabetes mellitus, and nonalcoholic fatty liver disease [Citation3–6]. Besides, OSAS may be one of the clinical signs for severe hypogonadism and disturbances in sexual function [Citation7].
Reactive oxygen species (ROS) are produced by healthy aerobic cells, which are increased in the state of any cellular damage. The functional levels of ROS facilitate many critical intracellular survival-signaling pathways. Besides, ROS itself may also cause inflammatory processes [Citation8].
Thiol is an organic compound with a critical role in preventing the formation of oxidative stress conditions within cells. The thiol groups of sulfur-containing amino acids such as cysteine and methionine in proteins are the primary target of ROS. Thiol groups in the presence of ROS are oxidized and transformed into reversible disulfide bonds. This conversion is the earliest indicator of radical-mediated protein oxidation. On the other hand, the dynamic thiol/disulfide homeostasis has vital roles in antioxidant defense, detoxification, apoptosis, regulation of enzyme activities, transcription, and cellular signal transduction mechanisms [Citation9].
The thiol/disulfide homeostasis was investigated in many disease conditions, including acute appendicitis in children, as a prognostic marker for myocardial infarction, and to predict colchicine resistance in Familial Mediterranean Fever (FMF) patients [Citation10–12]. It was reported that the anti-oxidant capacity decreased and anti-oxidant vitamins were lower in OSAS patients compared to the healthy controls [Citation13,Citation14].
This study aimed to evaluate the thiol/disulfide homeostasis in patients with OSAS, to compare the thiol/disulfide levels with the control group and to investigate their relationship with the severity of the disease.
Material and methods
Study design
The study was conducted in a case-control plan at Karabük University Faculty of Medicine (Departments of Chest Diseases and Medical Biochemistry) and Ankara Yıldırım Beyazıt University Faculty of Medicine (Department of Medical Biochemistry) between December 2017 and May 2018. Study reporting was done following the STROBE guidelines [Citation15]. All participants gave written individual informed consent to participate. The study protocol was approved by the Local Ethics Committee at Karabük University Medical Faculty.
Setting
Karabük University Faculty of Medicine is located in Karabük city, which is in the Black Sea region in northern Turkey. The Chest Diseases Department where the study patients were followed-up has a total of 27-bed capacity including three sleep laboratory units.
Participants
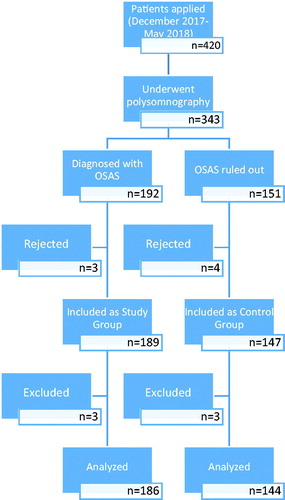
The study group was selected from patients who were admitted to the Karabük University Training and Research Hospital, Department of Chest Diseases and diagnosed with OSAS using polysomnographic analysis (n = 186, ). The patients were divided into three groups according to the severity of OSAS as mild, moderate, and severe. The patients had not received any OSAS treatment before joining the study. Participants in the control group consisted of 144 patients who underwent polysomnography due to some reasons but ruled out of having OSAS (). All participants were asked to complete a sociodemographic data form. The inclusion criteria for the study group were age over 18 years and having a diagnosis of OSAS. Patients with pregnancy and/or using anxiolytic or anti-psychotic medications were excluded from the study. Three patients from each group were excluded.
Variables
Dependent variables of the study were the serum total thiol (TT), native thiol (SH), and disulfide thiol (SS) levels. SS/SH, SS/TT, and SH/TT percent ratios were calculated.
The independent study variables were group (OSAS/control), age (years), BMI (kg/m2), gender (male/female), smoking (yes/no), diabetes mellitus (yes/no), hypertension (yes/no), coronary artery disease (yes/no), chronic obstructive pulmonary disease (yes/no), asthma (yes/no), apnea hypopnea index (AHI), hemogram parameters, serum fasting glucose (mg/dL), total cholesterol (mg/dL), triglyceride (TG) (mg/dL), high-density lipoprotein cholesterol (HDL) (mg/dL), low-density lipoprotein cholesterol (LDL) (mg/dL), urea (mmol/L), serum creatinine (mg/dL), total bilirubin (mg/dL), direct bilirubin (mg/dL), aspartate aminotransferase (IU/L), alanine aminotransferase (ALT: IU/L), serum albumin (g/dL), and ischemia modified albumin (IMA) (g/dL).
Bias
In the questionnaire, there was brief information about the research to ensure that the research data were obtained correctly, and participants were asked not to put their identities on the questionnaire form. To prevent bias, error checking and debugging were done after the data was entered into the computer.
Study size
The sample size was calculated based on the estimation of the SH levels [Citation16]. Using the one-way ANOVA analysis, a total sample of 216 cases are needed to compare the means between the four groups with an effect size of 0.228 (small–medium), an alpha error of 5%, and power of 80%. The standard deviation within each group was assumed as 60.0 [Citation17].
Quantitative variables
Height and weight measurements were made with the routinely used instruments of the hospital. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Based on the AHI, the severity of OSAS was classified as mild (AHI = 5–14), moderate (AHI = 15–30), and severe (AHI >30) [Citation18].
After 12-h fasting, peripheral venous blood samples were collected, centrifuged at 4000 rpm for 10 min., and stored at −80 °C. The samples were transferred on the same day of blood withdrawal via express cargo from Karabük to Ankara (distance: 221.9 km). It was ensured that the frozen samples did not melt during the transfer, and all samples were analyzed by the same researcher at one occasion after thawing. Glucose and lipid parameters were analyzed using the Roche Cobas c501 autoanalyzer (Roche Diagnostics, Berlin, Germany). All hemogram measurements were performed using the Sysmex XT-2000i Automated Hematology Analyzer (GMI, Ramsey, MN). Serum total and direct bilirubin levels were evaluated using the spectrophotometric end-point method Architect c16000 (Abbott Diagnostics, Lake Forest, IL). Plasma urea, creatinine, albumin, serum aspartate aminotransferase (SAT), and ALT levels were measured using the Cobas C111 autoanalyzer by Roche. IMA levels were determined by the albumin cobalt binding test (ACB) with a rapid colorimetric method developed by Bar-Or et al. [Citation19].
SH and TT levels were tested with a recently developed spectrophotometric method described by Erel and Neselioglu [Citation16]. Spectrophotometric analysis was performed by a Shimadzu UV-1800 spectrophotometer with a temperature controlled cuvette holder and an automated analyzer, Architect Plus C8000 (Abbott, Lake Bluff, IL). The concentrations of SS, and percentages of SS/SH, SS/TT, and SH/TT were calculated using the following formulas: SS levels (μmol/L) = (TT − SH)/2; SS/SH percentage (%) = (SS × 100)/SH; SS/ST percentage (%) = (SS × 100)/ST; and SH/TT percentage (%) = (SH × 100)/TT.
Statistical methods
The data were analyzed using the SPSS version 25.0 software (SPSS Inc., Chicago, IL). The results were presented as frequencies, percentages, means, and standard deviations (SD). The Shapiro–Wilk test was performed to test if the numerical variables in the patient and control groups were normally distributed. The independent samples t-test was used to compare the age, neutrophil, lymphocytes, hematocrit (HCT), mean corpuscular volume (MCV), total cholesterol, very low-density lipoproteins (VLDL), SH, and TT levels in the patient and control groups. The Mann–Whitney U test was used for other quantitative variables, and the Chi-Square test was used for categorical variables. Comparison of SH and TT means between groups including control, OSAS-mild, moderate, and severe were analyzed using the one-way ANOVA, followed by the post hoc Scheffe test. Comparison of SS level, SS/SH, SS/TT, and SH/TT between groups including control, OSAS-mild, moderate, and severe were analyzed using the Kruskal–Wallis test. Correlations were assessed using the Spearman’s test. A logistic regression analysis was performed to check for independent factors affecting OSAS. A p value of <.05 was considered statistically significant.
Results
Participants
The study comprised 186 patients with OSAS and 144 control subjects. The mean (±SD) age of the patients and control participants was 52.0 ± 11.5 years and 44.9 ± 13.2 years, respectively (t = 4.90; p < .001). Patients with OSAS had statistically significantly higher BMI (32.9 ± 7.5 kg/m2) than the control group (30.9 ± 6.6 kg/m2), t = 2.33, p = .021. The percentage of females was higher in the OSAS group than in the control group (69.9% (n = 130) and 52.6% (n = 60), respectively) (χ2 =9.07, p < .003). There were no statistically significant differences in smoking, coronary artery disease, chronic obstructive pulmonary disease, and asthma between the patient and control groups (p > .05). Comparison of the sociodemographic and clinical parameters of patients and controls are given in .
Table 1. Comparison of sociodemographic and clinical variables of the patients with obstructive sleep apnea syndrome and controls.
Descriptive data
Patients with OSAS had statistically significantly higher glucose (100.6 ± 37.5 mg/dL) than the control group (88.4 ± 30.8 mg/dL) (Z = 3.78, p < .001). Also, patients with OSAS had statistically significantly higher serum creatinine (0.87 ± 0.2 mg/dL) than the control group (0.79 ± 0.17 mg/dL) (Z = 3.41, p = .001). Besides, compared to the control group, patients with OSAS had significantly higher TGs (181.9 ± 87.1 mg/dL vs. 164.0 ± 88.7 mg/dL, Z = 2.22, p= .026) and serum albumin levels (3.6 ± 0.6 g/dL vs. 3.2 ± 0.4 g/dL, Z = 7.34, p < .001). There were no statistically significant differences in the white blood cell, urea, total cholesterol, and aspartate aminotransferase levels compared between the patient and control groups (p > .05). Comparison of the hemogram and biochemical parameters of the patients and controls are given in .
Table 2. Comparison of hemogram and biochemical variables of the patients and controls.
Outcome data
Compared to the control group, patients with OSAS had significantly lower SH (239.3 ± 56.3 μmol/L vs. 258.6 ± 65.3 μmol/L, t = 2.70, p = .007) and TT levels (273.2 ± 60.1 μmol/L vs. 292.9 ± 67.5 μmol/L, t = 2.64, p = .010). The difference in SH levels between the patients and controls was at borderline significance (16.9 ± 6.2 μmol/L vs. 17.2 ± 3.9 μmol/L, Z = 1.95, p = .051). Comparison of SH, TT, and SS level of the patients and controls are given in .
Figure 2. Comparison of native thiol, total thiol, and disulfide levels of the patients and controls.
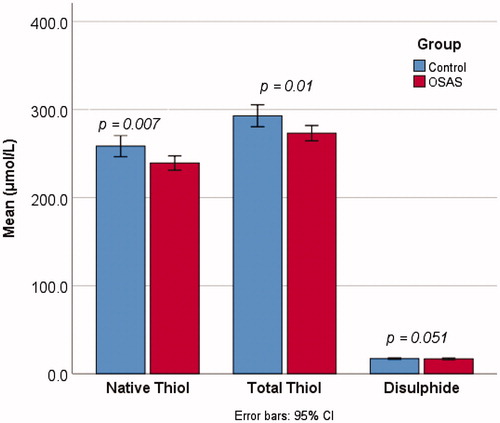
There were no statistically significant differences in the SS/SH, SS/TT, and SH/TT ratios between the patient and control groups (p>.05).
There was a significant difference in the SH levels between the groups as determined by the one-way ANOVA test (F = 6.15, p < .001). Post hoc Scheffe revealed that the SH was significantly higher in the control group (258.6 ± 65.3 μmol/L) compared to mild OSAS (221.6 ± 60.4 μmol/L, p=.001). Also, the TT levels between the groups were analyzed with one-way ANOVA, which demonstrated a significant difference (F = 6.10, p < .001). A post hoc Scheffe test revealed that the TT was significantly higher in the control group (292.9 ± 67.5 μmol/L) compared to the mild OSAS (254.9 ± 67.5 μmol/L, p=.001) as well as moderate and severe OSAS groups (p=.015, ).
Table 3. Comparison of thiol-disulfide homeostasis indicators and proportions in patients with obstructive sleep apnea syndrome and controls.
Plasma albumin levels were highly correlated with both SH (r = 0.66; p < .001) and TT levels (r = 0.69; p < .001). On the other hand, the plasma IMA levels showed a strong negative correlation with both SH (r = 0.50; p < .001) and TT levels (r = 0.53; p < .001) ().
Table 4. Correlations between serum albumin, ischemia-modified albumin, and thiol-disulfide indicators.
Using the enter method, a logistic regression analysis with study group (OSAS/controls) as the dependent variable, demonstrated that age (OR = 1.04), serum albumin (OR = 12.67), IMA (OR = 0.12), SH (OR = 0.81), and TT (OR = 1.17) were independent predictors of OSAS ().
Table 5. Logistic regression analysis computer output.
Discussion
Patients with OSAS had significantly lower SH and TT levels than the control group (p < .05) and borderline significantly lower SH than the control group (p=.051). There were no substantial differences in the SS/SH, SS/TT, and SH/TT proportions between the patient and the control groups (p>.05). On the other hand, the SH levels were significantly higher between the control group and severe OSAS as well as mild OSAS; the TT levels were significantly higher between the control group and mild OSAS.
The number of reports in the literature investigating the thiol/disulfide balance in patients with OSAS is limited [Citation20–22]. The available research provides a context for understanding the role of dynamic thiol/disulfide balance, the indicator of oxidative stress, in OSAS patients.
The study conducted by Dinc et al. [Citation21] reported that the serum SH and TT levels, as well as the SH/TT ratio, were significantly lower (p < .05) in OSAS patients compared to controls, and the SS levels, as well as the SS/SH and SS/TT ratios, were significantly higher (p < .05). Similarly, in our study, the SH and TT levels were higher, but the SS levels were lower in the OSAS patients compared to the controls. Additionally, the SS/SH, SS/TT, and SH/TT ratios were not different between the patients and the controls. In the study of Dinc et al. [Citation21], the SH levels and SH/TT ratio were significantly higher after continuous positive airway pressure (CPAP) therapy compared to before treatment (p < .05), while the SS levels, as well as the SS/SH and SS/total SH ratios, were significantly lower (p < .05). This shows the positive effect of correcting the low antioxidant capacity on the dynamic thiol/disulfide homeostasis.
OSAS leads to brain arousal, intrathoracic pressure changes, and recurrent incidents of hypoxemia, and reoxygenation. These processes activate pathways such as oxidative stress, sympathetic activation, inflammation, hypercoagulability, endothelial dysfunction, and metabolic dysregulation, which may predispose patients with OSAS to hypertension and atherosclerosis. OSAS is a common cause of systemic hypertension, and should be suspected in hypertensive individuals, especially those with resistant hypertension [Citation23]. In the study conducted by Go and Jones [Citation24], it was proven that abnormal thiol-disulfide homeostasis is implicated in the etiology of cardiovascular diseases. Also, OSAS and type 2 diabetes mellitus share common increased susceptibility to oxidative stress that may affect the vulnerable cells in different organs [Citation25].
Additionally, it was reported that although with unclear underlying mechanism, sexual problems are common among men with sleep apnea/hypopnea [Citation26]. OSAS has also been linked to impairments of vasoprotective function, resulting in erectile dysfunction in men. Thus, OSAS is recognized as a risk or even an etiological factor for erectile dysfunction [Citation27]. Aldemir et al. claimed a connection of increased oxidative stress markers in the aetiopathogenesis of erectile dysfunction [Citation28].
It has been assumed that the –SH groups of sulfur-containing proteins are related to maintaining oxidative equilibrium, which plays a critical role in the prevention of oxidative stress [Citation29]. Oxidation of free cysteine residues (SH) leads to the reversible formation of disulfide bonds (SS) between thiols and protein thiol groups, and this, in turn, can lead to abnormal thiol/disulfide hemostasis [Citation30]. Therefore, dynamic thiol/disulfide homeostasis is being increasingly accused of many disorders associated with ischemia.
We found that plasma albumin levels were also strongly correlated with both plasma ST and TT levels. In the study of Ateş et al. [Citation31], it was reported that the serum thiol levels were elevated in patients with chronic kidney disease (CKD). Similar results were published in a recent study conducted by Ozcan et al. [Citation32] in patients with sickle cell disease (SCD). They speculated that low serum native and TT levels may have resulted from low serum albumin concentrations in the CKD and SCD patients.
In contrast to the intracellular environment, TT levels in the plasma are at a lower concentration than inside the cells, and it mainly consists of human serum albumin thiols (approximately 80%) and to a less extent by low-molecular-weight thiols such as cysteine (Cys), cysteinylglycine, gamma-glutamylcysteine, glutathione, and homocysteine [Citation33]. However, in our study, serum albumin levels were higher in the patients with OSAS than in the control group, which suggests that low thiol levels are associated with OSAS, independent of serum albumin levels.
Some limitations of this study can be mentioned as follows. First, the study sample is not community-based; it is hospital-based. For this reason, there is no external validity of the results. Also, the controls were selected from patients who underwent polysomnography for reasons other than OSAS. Thus, the case and control groups were not matched for confounding factors such as age and gender.
Conclusion
As a conclusion, our results support the claim that decreased SH and TT levels are related to increased oxidative stress. On the other hand, impaired thiol balance may play a significant role in the pathogenesis of OSAS. However, this study was not planned to show causality between thiol balance and OSAS, which means that even OSAS can be a cause of impaired thiol balance cause. Further studies are necessary to elucidate this causal relationship.
Disclosure statement
The authors have no conflict of interest in this study.
References
- Epstein LJ, Kristo D, Strollo PJ. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276.
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239.
- Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240.
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360.
- Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384.
- Turkay C, Ozol D, Kasapoglu B, et al. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care. 2011;57:244–249.
- Shigehara K, Konaka H, Sugimoto K, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. 2018;21:99–105.
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95.
- Arioz DT, Camuzcuoglu H, Toy H, et al. Assessment of serum paraoxonase and arylesterase activity in patients with endometrial cancer. Eur J Gynaecol Oncol. 2009;30:679–682.
- Sivri S, Kasapkara HA, Polat M, et al. Dynamic thiol/disulphide homeostasis and its prognostic value in patients with non-ST elevation-acute coronary syndromes. Kardiol Pol. 2018;76:426–432.
- Elmas B, Yildiz T, Yazar H, et al. New oxidative stress markers useful in the diagnosis of acute appendicitis in children: thiol/disulfide homeostasis and the asymmetric dimethylarginine level. Pediatr Emerg Care. 2017. doi:10.1097/PEC.0000000000001339
- Omma A, Sandikci SC, Kucuksahin O, et al. Can the thiol/disulfide imbalance be a predictor of colchicine resistance in familial Mediterranean fever. J Korean Med Sci. 2017;32:1588–1594.
- Christou K, Moulas AN, Pastaka C, et al. Antioxidant capacity in obstructive sleep apnea patients. Sleep Med. 2003;4:225–228.
- Barcelo A, Barbe F, de la Pena M, et al. Antioxidant status in patients with sleep apnea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756–760.
- Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:1623–1627.
- Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–332.
- Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191.
- AASM. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American academy of sleep medicine task force. Darien (IL): AASM; 1999.
- Bar-Or D, Curtis G, Rao N, et al. Characterization of the Co2+ and Ni2+ binding amino-acid residues of the N-terminus of human albumin: an insight into the mechanism of a new assay for myocardial ischemia. Eur J Biochem. 2001;268:42–47.
- Ustundag Y, Demirci H, Balik R, et al. Thiol/disulfide homeostasis in pregnant women with obstructive sleep apnea syndrome. J Matern Fetal Neonatal Med. 2017;1–6. doi:10.1080/14767058.2017.1401995
- Dinc ME, Ozdemir C, Ayan NN, et al. Thiol/disulfide homeostasis as a novel indicator of oxidative stress in obstructive sleep apnea patients. Laryngoscope. 2017;127:E244–E250.
- Gul F, Muderris T, Yalciner G, et al. A novel method for evaluation of oxidative stress in children with OSA. Int J Pediatr Otorhinolaryngol. 2016;89:76–80.
- Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72.
- Go YM, Jones DP. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic Biol Med. 2011;50:495–509.
- Pallayova M, Banerjee D, Taheri S. Novel insights into metabolic sequelae of obstructive sleep apnea: a link between hypoxic stress and chronic diabetes complications. Diabetes Res Clin Pract. 2014;104:197–205.
- Taken K, Ekin S, Arısoy A, et al. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male. 2016;19:102–105.
- Li X, Dong Z, Wan Y, et al. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a meta-analysis. Aging Male. 2010;13:82–86.
- Aldemir M, Okulu E, Neşelioğlu S, et al. Evaluation of serum oxidative and antioxidative status in patients with erectile dysfunction. Andrologia. 2012;44:266–271.
- Bektas H, Vural G, Gumusyayla S, et al. Dynamic thiol–disulfide homeostasis in acute ischemic stroke patients. Acta Neurol Belg. 2016;116:489–494.
- Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972.
- Ateş İ, Özkayar N, Yılmaz F, et al. Oxidative stress level in patients with chronic kidney disease. Ortadogu Med J. 2018;10:45–50.
- Ozcan O, Erdal H, Ilhan G, et al. Plasma ischemia modified albumin levels and dynamic thiol/disulfide balance in sickle cell disease: case control study. Turk J Haematol. 2018;35:265–270.
- Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244–253.