Abstract
Objective: The association between erectile dysfunction (ED), hypogonadism, cardiovascular disease, and type 2 diabetes is well documented, but long-term data are limited. The aim of this study is to investigate effects of long-term testosterone therapy (TTh) with testosterone undecanoate in men with hypogonadism and ED.
Patients and methods: Observational, prospective registry of 805 hypogonadal men with different degrees of ED, evaluated by the International Index of Erectile Function – Erectile Function Domain. Four hundred and twelve patients underwent TTh, 393 patients served as controls, with an observation period up to 12 years.
Results: TTh led to substantial and sustained reduction of ED; improvement in erectile function was significant for each successive year until year 9. This was accompanied by improvements in cardiometabolic risk factors and urinary function throughout the 12-year follow-up period. Benefits of TTh were stronger for patients with moderate/severe ED than for patients with no/minor ED. Incidence of prostate cancer, major adverse cardiovascular events, and mortality were significantly lower in men on TTh compared with untreated men.
Conclusion: Long-term TTh for up to 12 years alleviates ED, improves cardiometabolic risk factors, and reduces prostate cancer. Patients must stay on TTh consistently for a long time to achieve maximum benefits of TTh.
Introduction
Erectile dysfunction (ED) is characterized by persistent difficulty to achieve and/or maintain an erection that is sufficient for intercourse. ED is the most common sexual dysfunction reported in urology practice and reason for assessing hypogonadism and – if indicated – initiation of testosterone therapy (TTh). The prevalence of ED increases with age; it affects 10% of young men in the 20–30 years age bracket but as many as 70% of men aged ≥70 years [Citation1–3]. Although the prevalence of ED increases with age, loss of erectile function does not seem to be caused by aging per se but rather by a number of contributing factors which, however, do increase with advancing age. Accumulating data show that type 2 diabetes (T2DM) is a stronger risk factor for ED than age [Citation4]. Obesity, hypertension, cardiovascular disease (CVD), depression, and lower urinary tract symptoms (LUTS) are common conditions that are also associated with ED [Citation2,Citation5–7]. ED and CVD share risk factors – such as obesity, hypertension, metabolic syndrome, diabetes, and smoking – and frequently coexist, with endothelial dysfunction believed to be the pathophysiologic link [Citation8]. Studies have demonstrated that ED in men with no known CVD often precedes the manifestations of CVD [Citation8,Citation9]. Accordingly, the Princeton Guidelines pointed out that a man with ED and no known CVD is a cardiac patient until proven otherwise [Citation10]. Accumulating data show that ED may be the earliest sign of subclinical CVD [Citation11]. A recent meta-analysis confirmed the association between ED and subclinical CVD, and underscored the importance of aggressive CVD risk assessment and management in men with ED [Citation12].
ED problems due to organic causes comprise up to 80% of cases, caused by vascular, neurogenic and/or endocrine disorders, or medications [Citation13]. Of all endocrine disorders, hypogonadism is the most common cause for ED [Citation14]. Testosterone (T) is involved in central as well as peripheral neural mechanisms of penile erection [Citation15]. T stimulates release of neurotransmitters in the brain that are responsible for the initiation of sexual desire and the biological process giving rise to an erection. T is also responsible for the development and function of the smooth muscle cells in the corpus cavernosum. Hence, T plays a crucial role in sexual drive (libido) and function (penile erection). A threshold level of T is an essential precondition for normal erectile function, although this T threshold likely varies between men.
The benefits of T therapy (TTh) in hypogonadal men with ED have been shown in a number of studies detailing the improvement of symptoms [Citation16–19]. However, there is a paucity of long-term studies. Therefore, the aim of this study is to fill this knowledge gap by conducting an analysis of hypogonadal men with ED of varying degrees, who had been treated with T undecanoate injections for up to 12 years.
Patients and methods
Patients
The observational, prospective, cumulative, ongoing registry study included 805 symptomatic hypogonadal men with a baseline total T level of ≤12.1 nmol/L (350 ng/dL). Patients who decided to start TTh were treated with injections of testosterone undecanoate (i.m. injections at 3-month intervals after an initial 6-week interval). Patients who opted against TTh served as controls.
Ethical guidelines formulated by the German Ärztekammer (German Medical Association) for observational studies in patients receiving standard treatment were followed. After receiving an explanation about the nature and the purpose of the study, all patients consented to be included in the registry and have their data analyzed.
ED severity was categorized based on the International Index of Erectile Function – Erectile Function Domain (IIEF-EF) questionnaire. A cutoff score of 22 (range 0–30) was set to differentiate patients with no or mild ED (“no/mild ED”) and mild-to-moderate-to-severe-to-very severe ED (“moderate/severe ED”).
Assessment and follow-up
Total T, IIEF-EF score, aging males’ symptoms (AMS) scale, anthropometric parameters (weight, waist circumference, BMI), lipids (total cholesterol, HDL, LDL, triglycerides) glycemic status (HbA1c, fasting glucose), blood pressure (systolic/diastolic), International Prostate Symptom Score (IPSS), and prostate parameters (prostate volume, PSA) were measured in the TTh group at each 3-monthly visit when patients came to the practice to receive T injections. In non-treated patients (controls), these parameters were measured at their biannual routine visits.
In the no/mild ED group, median follow-up was 11 (TTh patients) and 9 years (untreated patients), respectively. In the moderate/severe ED group, median follow-up was 6 (TTh patients) and 8 years (untreated patients), respectively. The longest follow-up was 12 years.
Statistical methods
Statistical methods have been described elsewhere [Citation20]. Briefly, data in both groups were averaged across each year, and yearly data were used to assess differences between groups while adjusting for possible confounding. Mean changes over time between groups were compared by a mixed effects model for repeated measures, with a random effect for intercept and fixed effects for time, group, and their interaction. To account for baseline differences between the two groups, changes were adjusted for the following covariates; age, weight, waist circumference, BMI, fasting glucose, blood pressure, lipids, and AMS.
Results
At baseline, no/mild ED (IIEF-EF score ≥22) was recorded in 298 patients (prevalence 37%), of which 154 underwent TTh and 144 served as the control group. Moderate/severe ED (IIEF-EF score <22) was recorded in 507 patients (prevalence 63%), of which 258 underwent TTh and 249 served as the control group.
In the no/mild ED group, mean age at baseline was 57.7 ± 6.6 and 64.1 ± 4.7 years in TTh patients and untreated patients, respectively. In the moderate/severe ED group, mean age at baseline was 58 ± 8 and 64 ± 5 years in TTh patients and untreated patients, respectively. The vast majority of patients in both groups were overweight or obese.
Comorbidities at baseline were primarily T2DM and CVD. The prevalence of T2DM was 34% and 42% in the no/mild ED group and the moderate/severe ED group, respectively. The prevalence of CVD was 24% in each ED group. Concomitant medications included alpha-blockers, 5-alpha reductase inhibitors (5-ARIs), statins, antidiabetics, antihypertensives, and phosphodiesterase-type 5 (PDE-5) inhibitors. A detailed description of baseline characteristics is presented in .
Table 1. Baseline characteristics, comorbidities and concomitant medication at baseline in no/mild ED and moderate/severe ED groups.
T, IIEF-EF, and AMS
In both ED groups, treatment with T undecanoate normalized total T after the first injection and kept total T in the normal range for the entire duration of the study. In untreated ED groups, total T progressively decreased during the entire observation period ().
Figure 1. Changes in total testosterone (nmol/L) in 298 hypogonadal men with no/ mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. Data are shown as least squares (LS) means ± standard errors (SE) after adjustment for waist circumference, weight, fasting glucose, systolic and diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, and AMS. Shaded areas show 95% confidence intervals.
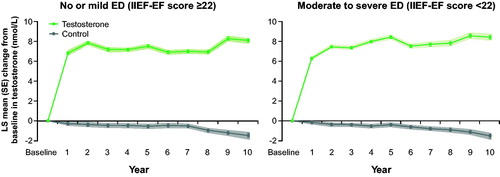
Changes in ED were consistent with the changes in total T; throughout the observation period, IIEF-EF scores increased steadily in both TTh groups, with a clear improvement in the no/mild ED group (+4 points) and an even greater improvement in the moderate/severe ED group (+11 points). Conversely, the untreated groups showed progressive worsening of ED over time ().
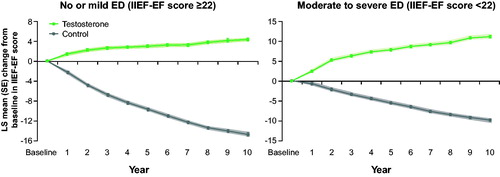
Figure 2. Changes in IIEF-EF in 298 hypogonadal men with no/ mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. Data are shown as least squares means ± standard errors after adjustment for waist circumference, weight, fasting glucose, systolic and diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, and AMS. Shaded areas show 95% confidence intervals.
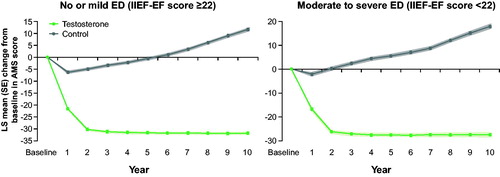
The AMS scores decreased by approximately 30 points in the TTh groups during the first 2 years of TTh and then remained constant (). In both control groups, AMS score slightly decreased in the first year of observation but then steadily increased during the remaining years of observation.
Figure 3. Changes in AMS in 298 hypogonadal men with no/ mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. Data are shown as least squares means ± standard errors after adjustment for waist circumference, weight, fasting glucose, systolic and diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, and AMS. Shaded areas show 95% confidence intervals.
Anthropometric parameters
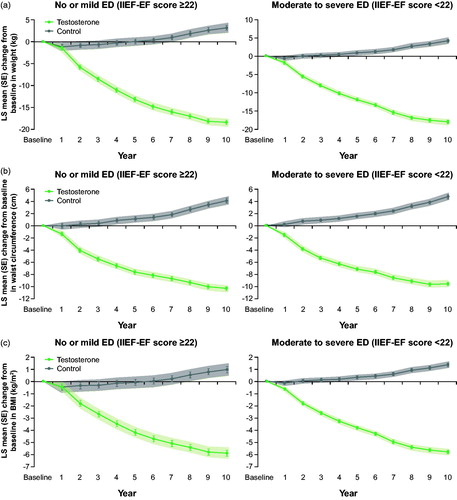
Both TTh groups experienced a marked weight loss throughout the entire observation period. Body weight was reduced by approximately 18 kg, waist circumference by 10 cm, and BMI by 6 kg/m2 (). In stark contrast, both untreated groups experienced weight gain over time (body weight of +3.2/+4.2 kg, waist circumference +4.1/+4.7 cm, BMI +1.0/+1.4 kg/m2).
Figure 4. (a) Changes in weight (kg) in 298 hypogonadal men with no/mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. (b) Changes in waist circumference (cm) in 298 hypogonadal men with no/mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. (c) Changes in BMI (kg/m²) in 298 hypogonadal men with no/mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. Data are shown as least squares means ± standard errors after adjustment for waist circumference, weight, fasting glucose, systolic and diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, and AMS. Shaded areas show 95% confidence intervals.
Metabolic parameters
In both TTh groups, there was an improvement in the lipid profile; total cholesterol, LDL and non-HDL cholesterol, and triglycerides decreased, while HDL increased. In both untreated groups, all lipid parameters progressively deteriorated further over time ().
Table 2. Changes at 10 years from baseline in testosterone-treated group (T-group) and untreated hypogonadal control group (control) and estimated difference between groups at 10 years, adjusted for baseline age, weight, waist circumference, fasting glucose, lipids, systolic and diastolic blood pressure, and AMS.
A similar pattern was seen in glycemic control. In both TTh groups, there was a considerable reduction in HbA1c and fasting glucose. In both untreated groups, a further deterioration of glycemic control was observed ().
Blood pressure
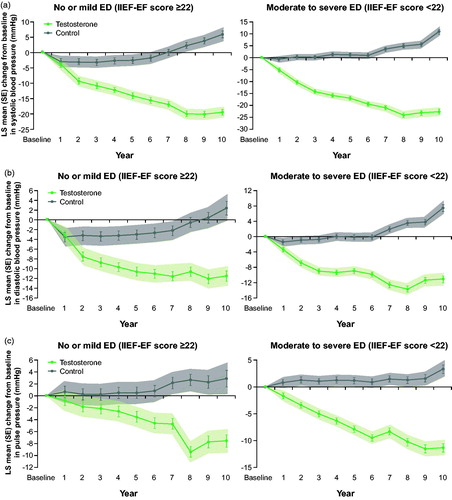
In both TTh groups, systolic and diastolic blood pressure was reduced by –19/–23 mmHg and –12/–11 mmHg, respectively. In both untreated groups, blood pressure increased by +6/+11 mmHg (systolic) and +2/+7 mmHg (diastolic) (). Pulse pressure was reduced by –8/–11 mmHg in the TTh groups, whereas in both control groups, an increase by 3 mmHg was noted ().
Figure 5. (a) Changes in systolic blood pressure (mmHg) in 298 hypogonadal men with no/mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. (b) Changes in diastolic blood pressure (mmHg) in 298 hypogonadal men with no/mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. (c) Changes in pulse pressure (mmHg) in 298 hypogonadal men with no/ mild ED (left panel) and 507 hypogonadal men with moderate/severe ED with or without long-term treatment with testosterone undecanoate. Data are shown as least squares means ± standard errors after adjustment for waist circumference, weight, fasting glucose, systolic and diastolic blood pressure, total cholesterol, HDL, LDL, triglycerides, and AMS. Shaded areas show 95% confidence intervals.
Urinary function and prostate parameters
In both TTh groups, IPSS total score was reduced by 4.6 points, whereas in the untreated groups the score increased by 2.4 and 2.8 points, respectively. Prostate volume increased in all groups, but this development was more pronounced in the untreated groups than in the TTh groups. In both TTh groups, PSA levels increased non-significantly from baseline (0.2 ng/mL). In contrast, in the untreated groups, PSA increased by 0.4 and 1.0 ng/mL in the no/mild ED group and the moderate/severe ED group, respectively (). The incidence of prostate cancer (PCa) was 3.2 versus 6.9% (p < .05) in the combined T groups and placebo groups, respectively.
summarizes changes from baseline to year 10 in the TTh groups and untreated groups, as well as estimated adjusted differences between TTh groups and untreated groups.
Mortality and major adverse cardiovascular events (MACE): In the TTh groups, no myocardial infarction (MI) or stroke occurred during the entire observation period. In the untreated groups, MI and stroke occurred in 18.9% and 14.9% of patients with moderate/severe ED, respectively, and 16% and 15.3% in patients with no/mild ED. In the TTh groups, death from all causes occurred in 6.5% and 2.3% of men with no/mild ED and moderate/severe ED, respectively. In the untreated groups, death occurred in 14.6% and 21.3% of men with no/mild ED and moderate/severe ED, respectively. Prostate cancer occurred in 13 men with moderate/severe who received TTh, and in 27 men in the untreated groups.
All-cause mortality and MACE during the entire observation period are summarized in .
Table 3. Major adverse events during the entire observation time up to 12 years.
Discussion
The main findings of this study are that long-term TTh with T undecanoate injections for up to 12 years substantially improves erectile function, anthropometric and cardiometabolic risk factors, PCa incidence and urinary function. It is notable that the benefits of TTh were even stronger in patients with moderate/severe ED at baseline than in patients with no/mild ED at baseline.
TTh significantly improved erectile function, as demonstrated by increased IIEF-EF scores by 5 points in the no/mild ED group and 12 points in the moderate/severe ED group. These results meet the requirement of minimal clinically important difference in IIEF-EF score from baseline of 4 [Citation21]. The larger improvement in patients with more severe ED is consistent with previous data [Citation21]. In the untreated groups, IIEF-EF decreased by 10 and 15 points in the no/mild EF and moderate/severe ED groups, respectively. This worsening of erectile function in the untreated groups is likely a consequence of aging related comorbidities. A similar significant and continuous improvement in IIEF-EF during TTh with T undecanoate was recently reported by Hackett et al. for an observation period of 4 years [Citation16]. To the best of our knowledge, the present study analyzing the effect of TTh on erectile function for 12 years is the longest reported observation to date. It is highly remarkable that erectile function continued to significantly improve for each successive year for 9 years. It was previously believed that maximal improvements in erectile function are achieved after 3–6 months of testosterone therapy [Citation22]. Based on this belief, several clinical guidelines recommend that men with borderline hypogonadism should be given a therapeutic trial of testosterone therapy for 3 months [Citation23], 6 months [Citation24], or 12 months [Citation25], to see if it works. Our data clearly show that a therapeutic trial of TTh for even 1 year is not enough to achieve maximal benefit of TTh on erectile function. An interesting finding in our study is that erectile function continued to improve for 9 years, even though improvement in AMS stabilized after 2 years. ED is one of the primary symptoms associated with hypogonadism [Citation26], and, therefore, improvement in erectile function is a major indicator of the effectiveness of testosterone therapy. Hence, it is critical for healthcare professionals to be informed that patients with hypogonadism who have been on testosterone therapy for 3–12 months but not experience the expected improvement in erectile function yet will likely do so if they stay on testosterone therapy without interruption for a longer time period.
ED has a detrimental impact on wellbeing and quality of life. We used the AMS questionnaire to assess the effect of TTh on physical, psychological, and sexual symptom relief over time, and overall quality of life (QoL). In the TTh groups, AMS scores decreased by 32 and 27 points in the no/mild ED group and the moderate/severe ED group, respectively. In contrast, in the untreated groups, AMS scores increased by 12 and 18 points in the no/mild ED group and the moderate/severe ED group, respectively. The large improvement in AMS scores paralleled the improvements seen in IIEF-EF scores and indicates a strong improvement of QoL in the TTh groups. The opposite was seen in the untreated groups.
A number of studies have shown that long-term TTh in men with hypogonadism and obesity leads to a substantial and sustained weight loss, and a reduction in waist circumference and BMI [Citation27–29]. This is in accordance with the findings in the present analysis. Both TTh groups experienced a continuous reduction in weight, waist circumference, and BMI, whereas patients in both untreated groups gained weight and waist size. The weight reduction in the TTh groups was progressive and sustained throughout the 12-year observation period, and occurred in parallel with the improvement in erectile function.
The prevalence of ED in men with T2DM is as high as 86% [Citation5,Citation30]. Men with T2DM have a 3.6-fold higher risk of developing ED compared with men without diabetes, and also tend to develop ED 10–15 years earlier than men without T2DM [Citation5]. The complex pathogenesis of diabetes and ED includes endothelial dysfunction, diabetic neuropathy, and hypogonadism. The prevalence of hypogonadism in men with T2DM mirrors that of ED; up to 81% of men with T2DM are hypogonadal [Citation31–34]. Accordingly, in 2018, the American Diabetes Association amended the Standards of Medical Care in Diabetes with the recommendation to routinely measure T levels in men with diabetes and symptoms of hypogonadism, such as ED [Citation35].
Our study confirms the high prevalence of T2DM in men with ED. At baseline, in men with no/mild ED, the prevalence of T2DM was 33.6%, whereas in men with moderate/severe ED, the prevalence of T2DM was 51.8%. In parallel with the alleviation of ED symptoms, the TTh groups achieved a significant and clinically important improvement in glycemic control. Remarkably, in the no/mild ED group and the moderate/severe ED group who received TTh, HbA1c decreased from 7.6% to 5.4% and 7.2% to 6.3%, respectively. The American Diabetes Association defines prediabetes as HbA1c 5.7–6.4% (39–46 mmol/mol). This means that TTh for 12 years downgraded T2DM to prediabetes. In a subgroup analysis including only men with T2DM who were treated with T undecanoate for up to 12 years, 12% of patients had remission of T2DM [Citation36]. In that analysis, patients who went into remission had a reduction in HbA1c from 8.3% at baseline to 5.7%, accompanied by a weight loss from 107 to 89 kg and reduction of waist circumference from 108 to 97 cm [Citation36]. These data are similar to our findings in the present study. In contrast, the untreated groups experienced a marked deterioration in glycemic control over time, consistent with the concept of diabetes as a progressive disease.
Accumulating clinical and epidemiological evidence suggests a direct link between ED and CVD. Endothelial dysfunction is a common pathophysiologic denominator that links ED with CVD, particularly in aging men with preexisting CV risk factors [Citation37–39]. Both ED and CVD share common risk factors such as hyperlipidemia, hypertension, smoking, obesity, and T2DM [Citation8]. ED may be considered an early manifestation of endothelial dysfunction irrespective of traditional CV risk factors, and serve as marker of vascular health and predictor of silent CVD [Citation40]. Accordingly, it is recommended that clinicians ask every man >40 years of age about ED, especially those men who are asymptomatic for signs and symptoms of CAD [Citation41].
Penile erection is regulated by a vascular process. The small penile vessels are highly sensitive to endothelial dysfunction and atherosclerosis; therefore, deterioration of erectile function may be indicative of systemic vascular disease [Citation39]. Based on this association, the American Urological Association (AUA) considers low T (often associated with ED) a risk factor for CVD and strongly recommends that all men with low T undergo clinical evaluation for atherosclerotic CVD [Citation42].
Pulse pressure (PP) is a marker for arterial stiffness, which in turn is related to endothelial dysfunction, and increased PP is associated with increased risk of CV and all-cause mortality [Citation43,44]. The association between ED and PP was confirmed in the present study. In the TTh groups, a clinically significant reduction in PP was observed in parallel with a clinically significant improvement in erectile function, whereas the untreated groups experienced progressive deterioration in both PP and ED.
This beneficial effect of long-term TTh on CVD-related major adverse events was clearly confirmed in the present study. In the TTh groups, there was no MI and no stroke. The lack of MACE in patients with moderate/severe ED is particularly remarkable considering their worse baseline health status and higher mortality risk. In contrast, in the untreated groups, MI and stroke occurred in 16% and 15% of patients with no/mild ED, and in 19% and 15% of patients with moderate/severe ED. This notable difference between TTh and untreated groups is likely explained by the improvement of CVD risk factors – obesity, hypertension, glycemic control, lipid profile – in men who had been treated with T undecanoate. Another study also reported a reduction in MACE and mortality after long-term treatment with T undecanoate [Citation45]. T deficiency may increase risk for ischemic events [Citation46]. TTh in stroke patients with hypogonadism and T2DM significantly reduced the incidence of recurrent stroke during a 5-year follow-up, and reduced mortality rates [Citation46]. Normalization of T levels may protect vascular function by reducing vasoconstriction and increasing coronary blood flow [Citation47]. The beneficial effects of TTh on vascular function merits further investigation.
Another highly remarkable finding in the present study is that there were no signs of negative impact on the prostate after long-term TTh with T undecanoate for 12 years. In fact, the incidence of PCa was more than twice as high in the untreated group compared to the TTh group (6.9% versus 3.1%). In accordance with our finding, several other studies have found that TTh may have a protective effect against PCa, especially high-grade PCa [Citation48–50]. Haider et al. reported that the incidence of PCa in men who had been treated with T undecanoate for up to 17 years was only 1.08% [Citation51]. This is much lower than the 7.35–9.6% PCa incidence rate in the general population of men who have never received TTh [Citation52,53]. Histological examination of prostate tissue before and after TTh confirms that TTh does not increase risk for prostate cancer development [Citation54]. Considering the strong association between hypogonadism and T2DM, it is not surprising that both T2DM [Citation55] and low testosterone [Citation56] are correlated with high-grade PCa, and that treatment with T undecanoate injections for up to 7 years in men with T2DM did not suggest increased risk of PCa [Citation57]. The present study adds to the growing body of evidence confirming the prostate safety of long-term TTh.
Conclusion
Long-term TTh with T undecanoate for up to 12 years significantly alleviates ED and markedly improves anthropometric obesity measures and metabolic risk factors. The new finding in the present study is that erectile function continues to significantly improve for 9 years during long-term continuous TTh.
Importantly, men who had been treated with T undecanoate had lower incidence of prostate cancer, major adverse cardiovascular events as well as mortality compared with untreated men. The present study underscores the importance for patients to stay on testosterone therapy consistently for a much longer time period than reported in randomized controlled studies in order to achieve maximum benefits of TTh in clinical practice.
Disclosure statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Additional information
Funding
References
- Sand MS, Fisher W, Rosen R, et al. Erectile dysfunction and constructs of masculinity and quality of life in the multinational Men's Attitudes to Life Events and Sexuality (MALES) study. J Sex Med. 2008;5:583–594.
- Laumann EO, West S, Glasser D, et al. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J Sex Med. 2007;4:57–65.
- Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007;120:151–157.
- Pallangyo P, Nicholaus P, Kisenge P, et al. A community-based study on prevalence and correlates of erectile dysfunction among Kinondoni District Residents, Dar Es Salaam, Tanzania. Reprod Health. 2016;13:140.
- Kouidrat Y, Pizzol D, Cosco T, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185–1192.
- Omland T, Randby A, Hrubos-Str⊘m H, et al. Relation of erectile dysfunction to subclinical myocardial injury. Am J Cardiol. 2016;118:1821–1825.
- Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol. 2014;65:968–978.
- Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract. 2013;67:1163–1172.
- Uddin SMI, Mirbolouk M, Dardari Z, et al. Erectile dysfunction as an independent predictor of future cardiovascular events. Circulation. 2018;138:540–542.
- Nehra A, Jackson G, Miner M, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012;87:766–778.
- Yao FJ, Zhang YD, Wan Z, et al. Erectile dysfunction is associated with subclinical carotid vascular disease in young men lacking widely-known risk factors. Asian J Androl. 2018;20:400–404.
- Osondu CU, Vo B, Oni ET, et al. The relationship of erectile dysfunction and subclinical cardiovascular disease: a systematic review and meta-analysis. Vasc Med. 2018;23:9–20.
- Chiurlia E, D’Amico R, Ratti C, et al. Subclinical coronary artery atherosclerosis in patients with erectile dysfunction. J Am Coll Cardiol. 2005;46:1503–1506.
- Corona G, Isidori AM, Aversa A, et al. Endocrinologic control of men’s sexual desire and arousal/erection. J Sex Med. 2016;13:317–337.
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol. 2007;52:54–70.
- Hackett G, Cole N, Mulay A, et al. Long-term testosterone therapy in type 2 diabetes is associated with decreasing waist circumference and improving erectile function. World J Mens Health. 2018;36:e33.
- Hackett G, Cole N, Saghir A, et al. Testosterone undecanoate improves sexual function in men with type 2 diabetes and severe hypogonadism: results from a 30-week randomized placebo-controlled study. BJU Int. 2016;118:804–813.
- Permpongkosol S, Khupulsup K, Leelaphiwat S, et al. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J Sex Med. 2016;13:1199–1211.
- Giltay EJ, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7:2572–2582.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199:257–265.
- Rosen RC, Allen KR, Ni X, et al. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60:1010–1016.
- Saad F, Aversa A, Isidori AM, et al. Onset of effects of testosterone treatment and time span until maximum effects are achieved. Eur J Endocrinol. 2011;165:675–685.
- Morales A, Bebb RA, Manjoo P, et al. Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. CMAJ 2015;187:1369. Appendix available at http://www.cmaj.ca/content/suppl/2015/10/26/cmaj.150033.DC1/15-0033-1-at.pdf (accessed Jan 10, 2016).
- Hackett G, Kirby M, Edwards D, et al. British Society for Sexual Medicine Guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14:1504–1523.
- Dean JD, McMahon CG, Guay AT, et al. The International Society for sexual medicine’s process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med. 2015;12:1660–1686.
- Wu FCW, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135.
- Yassin AA, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia. 2016;48:793–799.
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond). 2016;40:162–170.
- Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16:581–606.
- Walle B, Lebeta KR, Fita YD, et al. Prevalence of erectile dysfunction and associated factors among diabetic men attending the diabetic clinic at Felege Hiwot Referral Hospital, Bahir Dar, North West Ethiopia, 2016. BMC Res Notes. 2018;11:130.
- Biswas M, Hampton D, Newcombe RG, et al. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin Endocrinol (Oxf). 2012;76:665–673.
- Rezvani MR, Saadatjou SA, Sorouri S, et al. Comparison of serum free testosterone, luteinizing hormone and follicle stimulating hormone levels in diabetics and non-diabetics men – a case–control study. J Res Health Sci. 2012;12:98–100.
- Hackett GI, Kell P, Ralph D, et al. Biochemical hypogonadism in men with type 2 diabetes in primary care practice. 2009;9:226–231.
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769.
- Association American D. Updates to the standards of medical care in diabetes – 2018. Diabetes Care. 2018;41:2045–2047.
- Saad F, Yassin D, Dorsos A, et al. Most hypogonadal men with type 2 diabetes mellitus (T2DM) achieve HbA1c targets when treated with testosterone undecanoate injections (TU) for up to 12 years. Diabetes. 2017;66:A305. (abstract).
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:158–169.
- Tan HM, Tong SF, Ho CC. Men’s health: sexual dysfunction, physical, and psychological health-is there a link? J Sex Med. 2012;9:663–671.
- Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res. 2006;18:55–60.
- Azab SS, El Din Hosni H, El Far TA, et al. The predictive value of arteriogenic erectile dysfunction for coronary artery disease in men. J Sex Med. 2018;15:880–887.
- Miner M, Parish SJ, Billups KL, et al. Erectile dysfunction and subclinical cardiovascular disease. Sexual Med Rev. 2018. doi:10.1016/j.sxmr.2018.01.001
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA Guideline. J Urol. 2018;200:423–432.
- Zhao L, Song Y, Dong P, et al. Brachial pulse pressure and cardiovascular or all-cause mortality in the general population: a meta-analysis of prospective observational studies. J Clin Hypertens. 2014;16:678–685.
- Steppan J, Barodka V, Berkowitz DE, et al. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585.
- Traish AM, Haider A, Haider KS, et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a real-life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22:414–433.
- Morgunov LY, Denisova IA, Rozhkova TI, et al. Hypogonadism and its treatment following ischaemic stroke in men with type 2 diabetes mellitus. Aging Male. 2018. doi:10.1080/13685538.2018.1487932
- Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67:545–557.
- Yassin A, AlRumaihi K, Alzubaidi R, et al. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019. doi:10.1080/13685538.2018.1524456
- Wolf J, Keipert D, Motazedi H, et al. Effectiveness and tolerability of parenteral testosterone undecanoate: a post-marketing surveillance study. Aging Male. 2017;20:225–234.
- Yassin A, Salman M, Talib RA, et al. Is there a protective role of testosterone against high-grade prostate cancer? Incidence and severity of prostate cancer in 553 patients who underwent prostate biopsy: a prospective data register. Aging Male. 2017;20:125–133.
- Haider A, Zitzmann M, Doros G, et al. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year Median Followup of 3 Registries. J Urol. 2015;193:80–86.
- Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319.
- Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990.
- Efesoy O, Apa D, Tek M, et al. The effect of testosterone treatment on prostate histology and apoptosis in men with late-onset hypogonadism. Aging Male. 2016;19:79–84.
- Ohwaki K, Endo F, Shimbo M, et al. Comorbidities as predictors of incidental prostate cancer after Holmium laser enucleation of the prostate: diabetes and high-risk cancer. Aging Male. 2017;20:257–260.
- Tu H, Gu J, Meng QH, et al. Low serum testosterone is associated with tumor aggressiveness and poor prognosis in prostate cancer. Oncol Lett. 2017;13:1949–1957.
- Haider A, Cole N, Saghir A, et al. Long-term treatment with testosteroen undecanoate injections sustainably improves erectile function and metabolic control in hypogonadal men with type 2 diabetes mellitus (T2DM). 2015;193:e903–e903.
