?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
During 2015 in Poland, male-to-female ratio in age-adjusted cancer mortality rate amounted to 1.83, which is close to that observed in 1990 (1.94) and considerably more than in 1965 (1.38).
Data and methods
Nearest-neighbor matching and latent class model were estimated to assess gender gap in cancer prevention in 2006 and 2014. The analysis is based on nationally representative data from a two-wave survey carried out on a stratified random sample of adults.
Results
Even when controlling for socio-demographic characteristics, health status, and basic knowledge of cancer, three behavioral health characteristics are dramatically lower in men: uptake of preventive health care (ATE of –0.106), perceived caring for own health (–0.070), and fruit and vegetable intake (–0.034). Between 2006 and 2014 the gender gap in uptake of preventive health care and perceived caring for own health had increased, particularly in individuals aged over 40. The adjusted difference in leisure-time physical activity between men and women is near the significance threshold in individuals aged over 40.
Conclusion
The gender differences are higher for the behavioral component of cancer prevention than cognitive domains. Without modifying behavioral risk factors, gender gap in cancer mortality is not expected to close.
Introduction
In 2015 in Poland, 55,663 men and 44,938 women died due to cancer (C00–C97, D00–D09). Cancer is the second most common cause of death in Poland, exceeded only by heart disease. Eastern Europe shows the largest difference in life expectancy between men and women worldwide [Citation1]. Based on the population projection by Poland’s Central Statistical Office [Citation2], the number of men in 2035 is expected to be 1.2 million smaller than the corresponding number of women. In 2035, 1/3 of the country’s population aged over 80 will be male. In the current study, observed time trends in cancer mortality in men and women in Poland were compared.
Cancer mortality is determined by cancer incidence, time of finding cancer, and factors that influence the survival after diagnosis. Changing attitudes towards health issues is particularly important in cancer prevention, since cancer is considered a preventable disease and only 5–10% of all cancer cases are related to inherited genetic mutations [Citation3,Citation4]. The lifestyle factors for cancer include, among others, tobacco smoking, low fruit and vegetable intake, alcohol consumption, sun exposure, environmental pollutants, infections, stress, obesity, and physical inactivity [Citation3]. Gender differences in some of these areas were discussed in the current analysis. Observed gender disparities in cancer mortality have been considered to be the result of exposure factors, such as health care access and its utilization, excess body fat, viral infections, carcinogenic susceptibility, and hormones [Citation5]. In addition, it must be noted that there are substantial differences in smoking prevalence in men and women in the European Union (EU) countries, including Poland. According to the 2014 countrywide survey “Awareness of Cancer and its Prevention”, 31% of Polish men and 15% of Polish women are daily smokers. The size of the gender gap in tobacco use is similar among individuals in their early adulthood: 30% of Polish men aged 20–39 and 14% of Polish women aged 20–39 are daily smokers (data not shown).
The existence of gender gap is well known in a wide variety of areas, including income, education, employment, and entrepreneurship. In the EU countries, measures aimed at narrowing gender gap in socio-economic indicators were proposed. These measures are justified in the Partnership agreements on the European structural and investment funds which have been signed between the European Commission and each member state. Targeted actions were designed not only to narrow income, education, employment, and entrepreneurship inequalities between men and women, but also to change attitudes, including combating negative stereotypes about women’s role and the appropriate careers for them.
Previously, Hawkes and Buse [Citation6] noted that gender disparities in health are not properly addressed in policies and programs. Policy silence on the issue of male burden of ill health was also discussed by Baker et al. [Citation1]. In the current study, gender disparities in cancer prevention and cancer mortality in Poland were discussed. Since socio-demographic characteristics of men and women differ, the prevention outcomes were examined with covariates controlled by matching. The analysis is based on the results of a large-sample survey and registered mortality data.
Material and methods
In the current study, health-related characteristics in men and women were compared like with like using matching. The analysis was based on nationally representative data from a two-wave survey “Awareness of Cancer and its Prevention”, which was carried out on a stratified random sample of adults living in Poland, 48% of the sample was male. Size of the gender gap in cancer-preventive behaviors in 2006 and 2014 was compared. The study was contracted by the Maria Skłodowska-Curie Institute – Oncology Center and financed by the Ministry of Health of Poland within the National Program for Combating Cancer. The survey questions were focused on awareness of prevention advice of the European Code Against Cancer. The survey was conducted by computer assisted personal interview. The sample was randomly selected from the central electronic population record system (PESEL) using three-stage stratified random sampling.
In randomized experiments, the results in the two treatment groups are often directly compared e.g. by t-test. In non-randomized (observational) studies division into treatment group (or “ group”) and control group (or “
group”) is often influenced by individual’s characteristics. In the current study, these groups were defined by gender. They differ in the unobservable
and observable
which may cause hidden bias and overt bias. The latter is given by:
(1)
(1)
Matching is used to reduce or eliminate effects of confounding factors. Average treatment effect (ATE) was defined by Rosenbaum and Rubin [Citation7] to be the average difference between potential outcome for the treated and the potential outcome value for the not treated, whose unbiased estimator is:
(2)
(2)
However, one never observes
and
for the same individual.
If selection-on-observables assumption given by cannot be met, difference in
can be removed using matching. In the nearest-neighbor approach pairs are determined by using weighted function of covariates. This approach was first proposed by Rubin [Citation8,Citation9]. In the current study, a bias-corrected estimator was used for estimation of average treatment effects [Citation10]. Mahalanobis distance was used as a distance measure between subjects. There are two possible sources of bias, when estimating treatment effects: dimension problem and support problem. The first one is linked with unconfoundedness assumption of matching approach:
Common support assumption requires an overlap in the distribution of covariates between the groups under analysis:
Under these assumptions ATE can be estimated. Matching presented in the current paper was effective because the baseline characteristics are comparable between men and women.
There are many examples of identifying gender differences using matching, however, most studies have largely been published in the field of economics. There are few examples in the field of health sciences. Schiele et al. [Citation11] assessed differences across genders in the use of invasive procedures and 30-day mortality after acute myocardial infarction through the use of propensity score-matched analysis. After matching by characteristics, gender differences in the use of invasive procedures persisted, however, mortality differences became statistically insignificant. Weymann et al. [Citation12] evaluated gender differences in outcome after continuous-flow left ventricular assist device implantation and found that overall survival is comparable in male and female patients after matching using the baseline characteristics.
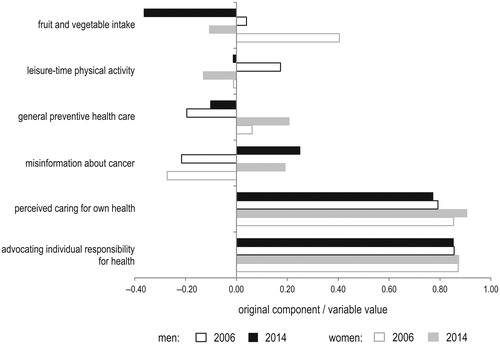
In the first part of the analysis of gender gap in cancer prevention, nearest-neighbor matching was applied in order to assess gender differences in the levels of the following health characteristics in 2006 and 2014 ():
fruit and vegetable intake,
leisure-time physical activity,
general preventive health care,
misinformation about cancer,
perceived caring for own health,
advocating individual responsibility for health.
Principal component analysis was used to extract the multidimensional measures of health characteristics designated by “a”–“d”. Principal components values were normalized to [0, 1] to ensure comparability of adjusted gender differences. shows most of the variance of the input variables is explained by the proposed components. The squared loadings represent the proportion of variance of the input variables explained by the principal component. Perceived caring for own health and advocating individual responsibility for health (“e”–“f”) were measured directly by two binary variables.
Table 1. Comparison criteria of disease prevention in men and women. Principal components loadings.
Men and women differ in socio-demographic aspects, such as age structure, education, place of residence, employment rate, and health condition. They differ also in terms of health status and life priorities. Matching enables to reduce or eliminate differences between data values that should be equal. In the comparison of men and women, a number of variables using matching estimation were controlled: age, education level, economic situation of the household, population size of the place of residence, smoking status, measured body mass index, suffering from frequent chronic diseases (including hypertension, coronary artery disease, diabetes, and osteoporosis), self-reported quality of communication with a physician, misinformation about cancer (excluding matching in “d”), perceived caring for own health (excluding matching in “e”), and advocating individual responsibility for health (excluding matching in “f”). Matching analysis was conducted with Stata version 13.0.
The matching analysis was followed by latent class analysis based on the previously listed health characteristics (). The latent classes are mutually exclusive and exhaustive subgroups that are homogenous or similar on certain criteria. The size of latent classes was compared in men and women in 2006 and 2014. The latent class model assumes that the relationship between observed variables can be explained by the latent variable.
Assume that the unobserved (or latent) variable X has latent classes; A and B are two observed (or manifest) variables with a level of
Let
denote the joint probability that an observation is in class
on variable A, in class
on variable B, and in class
on variable X. Let
denote the probability that an observation is in class
on variable A, given that the observation is in class
on variable X. The most basic latent class model in this situation is [Citation13]:
(3)
(3)
The model states that observed variables are conditionally independent of each other, given the class level on the latent variable:
(4)
(4)
Criteria used to select the final latent class model included Bayesian information criterion (BIC), Akaike information criterion (AIC), sample-size adjusted BIC, Lo-Mendell-Rubin test, bootstrapped likelihood ratio test, and entropy. BIC is a measure of model fit based on the −2 log likelihood statistic with penalization for additional classes and the total sample size. AIC penalizes by the total number of parameters only. The Lo-Mendell-Rubin test and the bootstrapped likelihood ratio test verify the hypothesis that reverting to a model with one less class than specified would improve model fit. Entropy measures how well individuals are assigned to latent classes with values ranging from 0 to 1 with values closer to 1 indicating better fit. The latent class analysis was performed using Mplus version 7.0.
Cancer mortality data for 1965–2015 come from Poland’s National Cancer Registry which processes information from death certificates collected by Poland’s Central Statistical Office. Age-adjustment of death rates was achieved by direct standardization and is based on the 2014 total resident population.
Results
Gender gap in cancer prevention
Adjusting for potential confounders, similar percentage of men and women claim an individual (not a state) should be responsible for their own health (). Unadjusted share of individuals advocating individual responsibility for health is also broadly consistent across genders and study waves (). Slightly fewer women than men tend to show low understanding of cancer both when adjusting for potential confounders and by direct comparison. By 2014, the adjusted difference in misinformation about cancer had become statistically insignificant. However, comparable levels of cancer awareness in men and women do not translate into similarities in cancer prevention behaviors, including fruit and vegetable intake, caring for own health, and uptake of preventive health care, both when comparing adjusted and unadjusted data.
Table 2. Gender gap identification by matching in adult individuals.
Both when adjusting for potential confounders and by direct comparison, gender differences in 2014 were highest in uptake of preventive health care. According to the 2014 survey “Awareness of Cancer and its Prevention”, men and women justify not performing preventive blood work (33.7% of men and 46.2% of women), tests of urine (36.8% and 49.4%), blood cholesterol (40.9% and 54.2%), blood sugar (40.8% and 54.8%), and not measuring blood pressure (35.4% and 48.0%) mainly by lack of physician’s instructions. However, lack of care about oneself is much more frequently reported as a factor of low preventive health care intake among men (31.8%–37.2% of men versus 20.5%–25.5% of women). A lack of time is similarly frequently reported reason in both genders (22.2%–20.3% of men versus 16.8%–19.7% of women). This is consistent with identified large gender gap in perceived caring for own health.
Between 2006 and 2014, the gender gap in adjusted and unadjusted uptake of preventive health care and perception of caring for own health had widened. This negative trend could be observed particularly in individuals aged over 40 years (). The opposite trend was observed for adjusted and unadjusted gender gap in fruit and vegetable intake. However, the gender gap in fruit and vegetable consumption had narrowed between 2006 and 2014 not due to improvement of dietary behaviors in men, but because of even more dynamic decrease in fruit and vegetable intake in women.
Table 3. Gender gap identification by matching in individuals aged over 40.
The gender disparities in health are in favor of men in the case of leisure-time physical activity. However, the difference in adjusted outcomes is considerable in younger individuals only (including individuals under 40 years of age: in 2006:
in 2014:
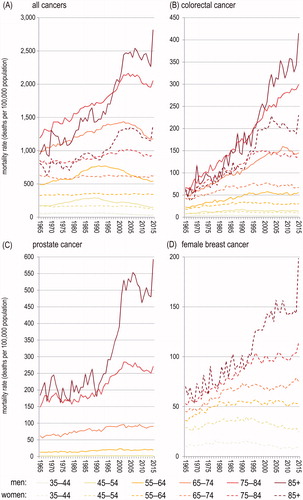
). Physical activity is much less gender-differentiated in individuals aged over 40 and the difference is near the significance threshold of 0.05 (). Cancer is an age-related disease, the incidence of most cancers increases with age dramatically. Most cancer cases occur after 40 years of age, particularly in the male population (). Hence, preventive role of high physical activity in overall male population on cancer is limited.
Considering the modeling results, it is important to remark being male currently negatively affects behavioral health characteristics, including fruit and vegetable consumption, participation in preventive health care, and carrying for own health, even when controlling for socio-economic characteristics, health status, and basic cancer knowledge. Gender gap in participation in preventive health care and self-reported caring for own health had widened between 2006 and 2014, particularly in individuals aged over 40, which is demonstrated by increased values of ATE. It should be stressed that the adjusted gender gap measured by ATE in 2014 was higher in individuals aged over 40 (representing a population at risk of cancer) than in the entire adult group for all comparison criteria but leisure-time physical activity. In 2006, the adjusted gender differences were smaller for most comparison criteria in individuals aged over 40 than in the entire adult group.
The matching results are largely consistent with those from latent class analysis. A four-class solution was judged best. According to AIC and BIC, sample-size adjusted BIC, Lo-Mendell-Rubin test, and parametric bootstrapped likelihood ratio test the four-class model is preferable (). The four-class solution shows high entropy (0.881), high repeatability and provides good interpretability.
Table 4. Relative size of latent classes and average score values.
The latent class 1 is characterized by the highest among the latent classes level of most behavioral health characteristics, including fruit and vegetable intake, uptake of preventive health care, and perceived caring for own health, very high level of basic knowledge of cancer, as well as near-average leisure-time physical activity and advocating individual responsibility for health. The size of this latent class was much smaller in men than in women in both 2006 and 2014. The latent class 2 differs most from the latent class 1 in relation to uptake of preventive health care, which is very low in the latent class 2 members. Latent class 2 members are also considerably less likely than latent class 1 members to report high fruit and vegetable intake and care for own health. The latent class 2 is characterized by the highest level of leisure-time physical activity, very high basic knowledge of cancer, frequent advocating individual responsibility for health, and near-average fruit and vegetable intake. The size of the latent class 2 relative to the latent class 1 was much larger in men than in women in both 2006 and 2014.
Very low basic knowledge of cancer is typical for the members of latent classes 3 and 4. In terms of odds ratios, they are also significantly more likely to refuse personal responsibility for their own health. The latent class 3 is characterized by lower level of all health determinants relative to the latent class 1. However, the uptake of preventive health care remains much above-average in the latent class 3 members. The latent class 4 is characterized by the lowest among the latent classes level of most behavioral health characteristics, including fruit and vegetable intake, preventive health care, and perceived caring for own health. There were more latent class 4 members in men than in women in both 2006 and 2014, but more latent class 3 members in women than in men. The gender gap in health is represented by underrepresentation of men in the latent class 1 relative to the latent class 2, as well as overrepresentation of them in the latent class 4 relative to the latent class 3.
Differences in cancer mortality
Observed all-site cancer mortality rates are higher in men in each age group but the individuals aged 35–44 (). Gender differences are notably higher for older age groups not only in absolute, but also in relative terms. Probability to die due to all-site cancer is more than twice higher for men than for women in the population aged over 70, also due to increasingly high prostate cancer mortality in men in their late adulthood (). In 2015 in Poland, the male-to-female ratio in age-adjusted cancer mortality rate amounted to 1.83, which is close to the corresponding value observed in 1990 (1.94) and significantly more than in 1965 (1.38). The dynamics differ between cancer sites (). In 1965–2015, the male-to-female ratio in age-adjusted lung cancer mortality rate (ICD-10: C33–C34, ICD-9: 162) had decreased from 6.23 to 2.03. In 2015 in Poland, the male-to-female ratio in age-adjusted colorectal cancer mortality rate amounted to 2.01, which is significantly more than in 1990 (1.47) and in 1965 (1.19).
Gender gap in age-adjusted cancer mortality in Poland is smaller in the population living in large cities (including city counties), as can be seen in . Cancer mortality in men is smaller in large cities compared to other areas, whereas in women the reverse is true. However, cancer mortality is higher in men than in women in each county (LAU 1). In 2010–2014, in city counties male-to-female ratio in age-adjusted mortality rate amounted to 1.68, while in other counties to 1.94.
Figure 3. Gender gap in cancer mortality in 1980–1984 and 2010–2014. Male-to-female ratio for age-adjusted cancer mortality rate.
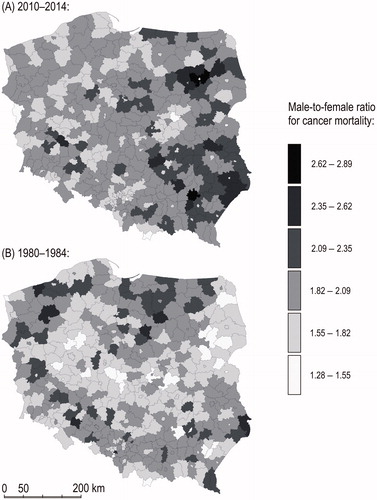
Gender disparities in cancer mortality are particularly high in Eastern Poland. This part of the country is known as Poland “B” due to its economic and social backwardness [Citation14]. In contrast, the capital metropolitan area is a bright spot on the map. Per capita, gross domestic product of the capital city of Warsaw in 2014 was 168% of the country’s average. In 2010–2014 both urban-rural and east-west polarization of gender gap in cancer mortality was higher than in 1980–1984.
Discussion
Cancer risk is increasingly greater among men. Men are currently less likely to follow healthy habits, including participate in preventive health care and have a healthy diet. When compared to a woman, a man of the same socio-economic characteristics, health status, and cancer knowledge is currently less likely to reduce most risk factors for cancer, according to the matching results. Top-down and bottom-up actions can and should facilitate a male shift to health-seeking behaviors. The use of current and retired professional athlete endorsements in health promotion campaigns could link masculinity to healthy life style. Review of possible health promoting interventions targeting men was presented by Robertson et al. and Anderson et al. [Citation15,Citation16].
Higher physical strength in men and the consequent social construction of man as the stronger sex is at the epicenter of the association between masculinity and lack of health orientation. These cultural restrictions are encapsulated in common sayings: “that is not masculine”, “real men do not behave like this” or “act like a man”. Unhealthy food consumption patterns [Citation17], drinking alcohol [Citation18], smoking tobacco [Citation19], delayed reporting of illness symptoms, late use of health care services [Citation20] have been linked to socially constructed notions of masculinity in various contexts. These risky health behaviors are responsible for most cancer deaths [Citation3].
Matching has not been used in the past to assess gender differences in cancer prevention. However, two-way statistical tests and regression models have been frequently used to evaluate gender disparities in health. Sach and Whynes [Citation21] based on a non-probabilistic sample of patients from Nottingham and Mansfield (England) found no difference in awareness of most cancer risk factors, including overweight, smoking tobacco, alcohol abuse, and lack of exercise. However, in a sample of the Danish population aged over 30 lower awareness of symptoms of cancer appeared to be significantly associated with being male [Citation22]. According to the presented results, gender differences in cancer awareness in Poland in 2014 were minor when adjusting for socio-economic characteristics and health status. This is consistent with the latent class prevalence for each gender shown in . As described previously, the most overrepresented in men is the latent class, which includes individuals of the highest basic cancer knowledge and support for individual responsibility for health, but considerably lower than in the latent class 1 uptake of preventive health care, fruit and vegetable intake, and perceived caring for own health. The latent class 1 is the most underrepresented subgroup in men. Some limitations of the current study should be noted. The data on cancer prevention are self-reported and retrospective which is inherently associated with residual confounding. However, one believes that matching ensured valid comparisons between men and women and controlled for some of the confounders.
Rosenberg and Hovland [Citation23] proposed a tripartite view of attitude which can be used to explain gender gap in cancer prevention analyzed in the study. Within this conceptualization, an attitude contains cognitive, affective, and behavioral components. Cognitive differences in cancer prevention are relatively low, as discussed in the preceding paragraph. Affective differences appear to be large, according to the matching estimation results and the existing body of evidence. Men do not perceive themselves at risk for health problems [Citation24] and seek help less often [Citation25]. High differences between men and women apply to the behavioral component of attitude towards cancer prevention. Previous studies have shown that women use much more preventive health care services than men do [Citation26,Citation27]. In New York, Maryland (the United States), and Puerto Rico, 41% adult men have never had a cancer screening in the past compared to 5% adult women who had never had a screening. Particularly high differences in uptake of screening in men and women were found for skin cancer [Citation27], of which incidence and mortality are higher in men.
Differences in uptake of health care are largely shaped by health policy. By 2030, the number of deaths due to prostate cancer in Poland will be higher than the corresponding number for breast cancer [Citation28]. At the same time, urology has never been listed among priority areas of medicine by the Minister of Health of Poland. As of 2019 according to Poland’s Minister of Health’s regulation, there are 20 of them. 31% of Polish men and 15% of Polish women are daily smokers. Przewoźniak et al. [Citation29] designed a Delphi study on Poland’s National Tobacco Control Program for 2018–2024 (NTCP) and Poland’s Cancer Control Strategy for Poland in 2016–2024 (CCSP). 24 experts in the field of tobacco and cancer control ranked according to their importance in these documents seven target groups: individuals aged 40–59, children and youth, unemployed individuals, poorest individuals, lower education groups, men, and women. Seven ranks were assigned to the seven target groups with rank 1 corresponding to the most important group and 7 to the least important group according to the documents. Overall ranks for men and women were equal to 4.92, 3.46 in NTCP and 4.75, 3.25 in CCSP, respectively.
Fruit and vegetable intake is lower among men in most countries, including the EU countries, the United Kingdom, and the United States [Citation30–32]. This was confirmed by direct comparison, statistical matching, and latent class analysis in the current study. In the United States, the frequency of consumption of fruits and vegetables was also found to decline more dynamically in men than in women in 1994–2005 [Citation33]. In most cultures, fruits and vegetables are considered to be more suitable for women, while male identity is currently verified though eating energy-dense foods, including meat [Citation34].
Regardless of age, Polish women are on average less active than Polish men, however, time spent on leisure-time physical activity decreases with age more dynamically among men than among women. According to the presented matching results, when controlling for socio-economic characteristics, health status, and basic cancer knowledge, the difference in leisure-time physical activity in men and women aged over 40 is near the significance threshold. The same age-related difference in time spent on leisure-time physical activity was observed in the previous study based on population-based sample of adults living in the southern Brazilian city of Pelotas [Citation35]. In 2015, the governments of the European states were invited to implement 42 measures to narrow gender inequalities between men and women in sport, having the aim of the full involvement of women and girls in every aspect of sport [Citation36].
In conclusion, taking preventive measures against cancer is strongly differentiated by gender. Gender differences in most health characteristics persisted after matching by socio-economic variables, health status, and basic cancer knowledge. This means that being male or female currently affects health behaviors by itself. Without modifying behavioral risk factors, which play a crucial role in cancer etiology, gender gap is not expected to close. Widening of the gender gap in health between 2006 and 2014 will translate into high male-to-female ratio in cancer mortality in the future.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Baker P, Dworkin SL, Tong S, et al. The men's health gap: men must be included in the global health equity agenda. Bull World Health Organ. 2014;92:618–620.
- Central Statistical Office (Poland). Population projection 2014–2050. Warsaw: Statistical Analyses and Studies; 2014.
- Anand P, Kunnumakara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116.
- Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23:6445–6470.
- Cook MB, McGlynn KA, Devesa SS, et al. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–1637.
- Hawkes S, Buse K. Gender and global health: evidence, policy, and inconvenient truths. Lancet. 2013;381:1783–1787.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
- Rubin DB. Matching to remove bias in observational studies. Biometrics. 1973;29:159–183.
- Rubin DB. Assignment to treatment group on the basis of a covariate. J Stat Educ. 1977;2:1–26.
- Abadie A, Imbens GW. Bias-corrected matching estimators for average treatment effects. J Bus Econ Stat. 2011;29:1–11.
- Schiele F, Meneveau N, Seronde MF, et al. Propensity score-matched analysis of effects of clinical characteristics and treatment on gender difference in outcomes after acute myocardial infarction. Am J Cardiol. 2011;108:789–798.
- Weymann A, Patil NP, Sabashnikov A, et al. Gender differences in continuous-flow left ventricular assist device therapy as a bridge to transplantation: a risk-adjusted comparison using a propensity score-matching analysis. Artif Organs. 2015;39:212–219.
- Goodman LA. Latent class analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied latent class analysis. New York: Cambridge University Press; 2002. p. 3–55.
- Pietrzak B, Wilk J, Chrzanowska M. Economic situation of eastern Poland and population migration movement. Metody Ilościowe w Badaniach Ekonomicznych. 2013;14:148–157.
- Robertson LM, Douglas F, Ludbrook A, et al. What works with men? A systematic review of health promoting interventions targeting men. BMC Health Serv Res. 2008;8:141.
- Anderson C, Seff LR, Batra A, et al. Recruiting and engaging older men in evidence-based health promotion programs: perspectives on barriers and strategies. J Aging Res. 2016;2016:1.
- Gough B. 'Real men don't diet': an analysis of contemporary newspaper representations of men, food and health. Soc Sci Med. 2007;64:326–337.
- de Visser ROD, Smith JA. Alcohol consumption and masculine identity among young men. Psychol Health. 2007;22:595–614.
- Odimegwu C, Pallikadavath S, Adedini S. The cost of being a man: social and health consequences of igbo masculinity. Cult Health Sex. 2013;15:219–234.
- Maclean A, Sweeting H, Hunt K. 'Rules' for boys, 'guidelines' for girls: Gender differences in symptom reporting during childhood and adolescence . Soc Sci Med. 2010;70:597–604.
- Sach TH, Whynes DK. Men and women: beliefs about cancer and about screening. BMC Public Health. 2009;9:431.
- Hvidberg L, Pedersen AF, Wulff CN, et al. Cancer awareness and socio-economic position: results from a population-based study in Denmark. BMC Cancer. 2014;14:581.
- Rosenberg MJ, Hovland CI. Cognitive, affective and behavioral components of attitudes. In: Rosenberg MJ, Hovland CI, editors. Attitude organization and change: an analysis of consistency among attitude components. New Haven: Yale University Press; 1960. p. 112–163.
- Courtenay WH. Engendering health: a social constructionist examination of men’s health beliefs and behaviors. Psychol Men Masculin. 2000;1:4–15.
- Mansfield AK, Addis ME, Mahalik JR. “Why won’t he go to the doctor?”: the psychology of men’s help seeking. Int J Mens Health. 2003;2:93–110.
- Vaidya V, Partha G, Karmakar M. Gender differences in utilization of preventive care services in the United States. J Womens Health (Larchmt). 2012;21:140–145.
- Davis JL, Buchanan KL, Katz RV, et al. Gender differences in cancer screening beliefs, behaviors, and willingness to participate: implications for health promotion. Am J Mens Health. 2012;6:211–217.
- Czaderny K. High prostate cancer mortality in Poland. A spatial, temporal, and structural analysis. Przegl Epidemiol. 2018;72:235–246.
- Przewoźniak K, Łobaszewski J, Koczkodaj P. Are tobacco and cancer control strategies in Poland consistent, harmonized and comprehensive? Results of two-round Delphi study. Tob Prev Cessation. 2018;4:A165.
- Prättälä R, Paalanen L, Grinberga D, et al. Gender differences in the consumption of meat, fruit and vegetables are similar in Finland and the Baltic countries. Eur J Public Health. 2007;17:520–525.
- Baker AH, Wardle J. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40:269–275.
- Emanuel AS, McCully SN, Gallagher KM, et al. Theory of planned behavior explains gender difference in fruit and vegetable consumption. Appetite. 2012;59:693–697.
- Fagerli RA, Wandel M. Gender differences in opinions and practices with regard to a “healthy diet”. Appetite 1999;32:171–190.
- Blanck HM, Gillespie C, Kimmons JE, et al. Trends in fruit and vegetable consumption among U.S. men and women, 1994-2005. Prev Chronic Dis. 2008;5:A35.
- Azevedo MR, Araújo CLP, Reichert FF, et al. Gender differences in leisure-time physical activity. Int J Public Health. 2007;52:8–15.
- Recommendation CM/Rec(2015)2 of the Committee of Ministers to member States on gender mainstreaming in sport. Adopted by the Committee of Ministers on 21 January 2015 at the 1217th meeting of the Ministers’ Deputies