Abstract
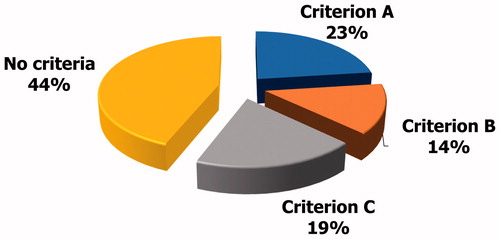
The Italian law 40/2004 allows the use of assisted reproduction techniques only if there are no other effective therapeutic approaches to overcome infertility. According to article 4 paragraph 1, the impossibility of removing the otherwise impeding causes to achieve a pregnancy must be ascertained before the couple undergoes assisted reproduction techniques. On this premises, we sought to evaluate the percentage of couples who underwent or were addressed to assisted reproduction techniques despite a known and potentially treatable male infertility factor in fertility centers in the city of Catania, Italy. To accomplish this, andrologists, urologists and endocrinologists were asked to report the number of couples already addressed to assisted reproduction techniques which they counseled in the trimester April–June 2018 having a under 35-year-old female partner and at least one among the following untreated conditions: (A) oligoasthenoteratozoospermia and FSH <8 mIU/ml, (B) third-degree varicocele (mono or bilateral form), and (C) leukocytospermia or urogenital infections. Of the 320 enrolled couples, 75 (23%) met the criterion A, 45 (14%) the criterion B, and 62 (19%) the criterion C. More than a half couples were addressed to assisted reproduction techniques despite a potentially treatable male infertility factor.
1. Background
According to the World Health Organization (WHO) and the American Fertility Society, infertility is defined as the failure to achieve pregnancy in 12–24 months of unprotected sexual intercourse [Citation1]. It has been esteemed to affect about the 15% of couples in reproductive age in Western countries. Epidemiological studies from the general population report a chance of conception of about the 80–85% in 12 months of sexual intercourse. In the absence of a male factor of infertility, the remaining couples will achieve pregnancy in 6 years. In case of male factor of infertility, only the 22–35% of couples will achieve pregnancy in 12 years [Citation1].
Data from the Italian Institute of Health indicates that, among infertile couples, a male factor of infertility is identified in the 35% of cases, a female factor in 35% of cases, whereas both a male and a female factors can be found in the 15% of cases. The etiology of the couple’s infertility remains unknown in the remaining 15% of cases [Citation1].
Male infertility is characterized by abnormal conventional sperm parameters (concentration, motility, morphology), resulting in oligozoospermia (sperm count <15 × 106/ml), asthenozoospermia (progressive motility <32% and total motility <40%) and/or teratozoospermia (normal forms <4%), although bio-functional sperm parameters (DNA integrity, mitochondrial function, chromatin compactness, sperm apoptosis) may also be affected [Citation2]. Male infertility recognizes (i) pre-testicular causes, that have a prevalence of the 10% and account for all the conditions interfering with the function of the hypothalamus–pituitary–testis axis, resulting in decreased gonadotropins and/or testosterone secretion and in a consequent reduced sperm production (e.g. hypogonadotropic hypogonadism, hypopituitarism, hyperprolactinemia, etc.); (ii) testicular causes, showing a prevalence of the 75% and including all the conditions responsible for a primary testicular failure and impaired spermatogenesis (e.g. varicocele, cryptorchidism, testicular tumor, Klinefelter syndrome, etc.) and (iii) post-testicular causes, that, overall, have a prevalence of the 15% and consist of all the conditions damaging the anatomy and/or the health of the sperm excretory ducts and the male accessory glands, thus impairing the sperm quality or the ejaculation (e.g. male accessory gland infection/inflammation (MAGI), anejaculation, acquired or congenital obstruction, etc.) [Citation1]. In addition, life style and environmental factors have been recognized to influence sperm parameters [Citation3–6].
Most of the causes of male infertility are treatable and their removal can improve the sperm quality. In agreement, according to the Law no 40 of 19th February 2004 (Law 40/2004), the diagnosis and treatment of male factors of infertility has to be performed before addressing the couple to assisted reproduction techniques (ART) [Citation7].
The use of ART is widespread in our country. The number of operative ART centers in Italy has been esteemed to be up to 360 in the year 2016, with a number of treated couples equal to 77.522 (Ministry of Health, 28 June 2018). ART comprises first-level techniques, such as intrauterine insemination (IUI), where the embryo is formed within the uterus, second- and third-level techniques (e.g. in vitro fertilization (IVF) and intracytosplasmic sperm injection (ICSI)), which differ from the previous for the in vitro formation of the embryo.
The article 1 paragraph 2 of the Italian Law 40/2004 allows the use of ART only if there are no other effective therapeutic methods to overcome the causes of infertility [Citation7]. According to article 4 paragraph 1, the impossibility of removing the otherwise impeding causes to achieve a pregnancy must be ascertained before the couple undergoes ART [Citation6]. Moreover, article 4 paragraph 2 establishes the principle of graduality: the less invasive treatment must be chosen, avoiding, at first, interventions with greater technical and psychological invasiveness [Citation7]. This principle should be applied not only to choose the type of technique to be used (first, second or third level), but also for the treatment of male factors of infertility.
According to the latest report of the Ministry of Health to the Italian Parliament, the success rate after ART in Italy is about 18% (Ministry of Health, 28 June 2018). This success rate has remained unchanged over the years. In the attempt of better understand the reasons of the lack of improvement of the success rate after ART, we evaluated the percentage of couples, with woman under 35 years, who underwent ART or who have received indications to perform ART despite a known and potentially treatable male infertility factor, in andrology, urology and endocrinology centers in the city of Catania (Italy).
2. Methods
2.1. Patient selection
This was a multi-center cross-sectional survey performed in the trimester April-June 2018. Andrologists, urologists and endocrinologists working in the city of Catania (Italy) were invited to report the number of couples with women under 35, looking for their counseling in the trimester April-June 2018, already addressed to ART, whose male partner met at least one of the following criteria: (i) Criterion A: untreated oligoasthenoteratozoospermia (OAT) with follicle-stimulating hormone (FSH) values <8 mIU/ml, in the absence of concomitant varicocele and/or leukocytospermia; (ii) Criterion B: Untreated mono or bilateral third-degree varicocele, in the absence of other relevant andrological co-morbidities (high FSH, leukocytospermia or current urogenital infection); (iii) Criterion C: Leukocytospermia or urogenital infections not treated with antibiotic/anti-inflammatory therapy or without proved eradication.
Sperm parameters had be analyzed according to the 2010 WHO guidelines (WHO, 2010) and the occurrence of varicocele with the scrotal Doppler ultrasound examination. In addition, evaluation for urogenital infection had to be performed according to the currently adopted diagnostic criteria that are detailed elsewhere [Citation8].
2.2. Statistical analysis
Prevalence differences are reported throughout the study. Statistical analysis was performed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
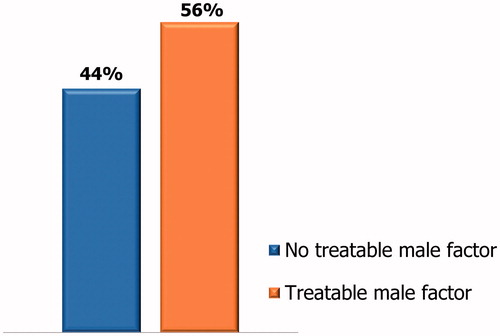
A total of 320 couples who underwent ART or received indications to ART performed andrological, urological or endocrinological outpatient visit in the period April–June 2018. Among them, 75/320 (23%) met the criterion A, 45/320 (14%) met the criterion B, 62/320 (19%) met the criterion C (). Overall, 182/320 couples (56%) met at least one of the above-mentioned criteria ().
4. Discussion
The present data show that in more than half couples with women under 35 addressed to ART there was a potentially treatable male factor of infertility, such as untreated OAT, varicocele and leukocytospermia or urogenital infection. The treatment of these conditions might influence the ART outcome.
Administration of FSH represents a hormonal therapeutic strategy that can be adopted in oligozoospermic patients with gonadotropin serum levels within the normal range [Citation2]. Indeed, according to the latest Cochrane review on this topic, given its relevant role on spermatogenesis, FSH administration is able to improve sperm quality and the spontaneous pregnancy rate [Citation9]. Different preparations are available, such as the purified [extracted from urine of post-menopausal women and called human-purified FSH (hpFSH)] [Citation10] and synthetized ones [produced by recombinant in vitro technology (rhFSH)] [Citation11]. The Italian Society of Andrology and Medicine of Sexuality (SIAMS) suggests the effectiveness of FSH administration for the amelioration of sperm concentration, motility, DNA fragmentation and pregnancy outcome, suggesting its prescription in normogonadotropic patients with idiopathic OAT to improve all these parameters [Citation12]. Therefore, this therapeutic option should be considered prior to ART. We have recently suggested that four FSH therapy protocols are feasible for patients with male infertility [Citation13].
Varicocele is a varicose enlargement of the veins of the pampiniform plexus causing an abnormal testicular venous drainage which is responsible for increased testicular and scrotal temperature, reflux of renal and adrenal metabolites along the spermatic vein, pool of venous blood and hypoxia into the testis [Citation14]. According to the Ministry of Health, it is considered one of the main acquired causes of male infertility, affecting about the 40% of infertile patients [Citation15]. The evidence clearly shows the negative effect of varicocele on the sperm parameters [Citation16,Citation17], including sperm DNA fragmentation [Citation18]. Its removal can improve the sperm quality [Citation19,Citation20]. Meta-analytic data confirm the benefit of varicocele repair in infertile patients before ART [Citation21]. Furthermore, varicocele repair, by improving the sperm parameters, may decrease the level of ART needed to achieve successful pregnancy in couples seeking fertility [Citation22].
A large body of literature has clearly shown the negative impact of leukocytospermia on sperm quality, mostly due to the increased levels of reactive oxidative species that cause sperm peroxidative damage and DNA fragmentation [Citation8]. The adverse consequences of leukocytospermia on ICSI outcome have already been established [Citation23]. Accordingly, the American Society of Reproductive Medicine (ASRM) has included the quantification of sperm leukocytes in the diagnostic evaluation of infertile patients [Citation24]. In addition, MAGI can also affect both conventional and biofunctional sperm parameters [Citation8,Citation25]. In the light of such evidence, an accurate diagnostic work-up should be performed to rule out the presence of leukocytospermia and/or MAGI in the infertile patient. A targeted therapeutic strategy should be accomplished in case of their occurrence before addressing the couple to ART.
As discussed above, male infertility factors often reflect on sperm DNA integrity. This latter heavily influence the ART success, as supported by meta-analytic data [Citation26,Citation27]. Indeed, a high sperm DNA fragmentation has a detrimental effect on IVF/ICSI outcome, with decreased pregnancy rate and increased miscarriage rate [Citation26]. On this account, treatment of male infertility factors, by improving sperm DNA integrity, positively influences the ART result.
The Italian Ministry of Health recently reported a success rate after ART of the 18% (Ministry of Health, 28 June 2018), which has remained unchanged over the years. According to the data presented in this study, in more than the half of cases, couples with women under 35 years are addressed to ART despite a potentially treatable male infertility factor. After an adequate diagnostic work-up, it is possible to establish the etiology of male infertility in 70–80% of the cases and, in almost half of them, it is also possible to start a specific treatment. Results of specific treatment could be better than those of ART [Citation28] and might contribute to increase ART success rate. Furthermore, do not treat a known factor of infertility violates articles 1 and 4 of the law number 40 of 19th February 2004 as well as the principle of graduality enshrined in the same law [Citation7]. In addition, ART is an extremely expensive procedure. In an era when the cost-effectiveness of healthcare has become essential in all areas of medicine, do not treat a known factor of male infertility is both unethical and uneconomical [Citation29].
In conclusion, we found that in half couples with women under 35 and previous indications to ART, subsequently reevaluated by andrologists, urologists and endocrinologists, there was a potentially treatable male factor of infertility, thus violating the principle of graduality in the application of ART, as established by the Law 40/2004 [7]. A minimal andrological evaluation of the male partner should be carried out routinely in all infertile couples. It should at least include a careful clinical history, with particular attention to the genital and reproductive system, and two semen analyses. If the initial evaluation shows an abnormal clinical history and/or altered sperm parameters, a thorough andrological evaluation is mandatory [Citation30]. Results of specific treatment could be better than those of ART [Citation28] and might contribute to increase ART success rate.
Consent for publication
Not applicable.
Ethics
The survey has been conducted in accordance with the principles expressed in the Declaration of Helsinki.
Author’s contribution
YD conceived and designed the study and carried out the analysis and interpretation of data. AEC drafted the article and revised it critically. RAC participated in data acquisition, analysis and interpretation of data. ESV participated in data acquisition. RC wrote the article. FV participated in data acquisition. GS participated in data acquisition. PP participated in data acquisition. FL participated in data acquisition. BG participated in data acquisition. SLV conceived and designed the study and carried out the analysis of data, participated in data acquisition and revised the manuscript critically. All authors read and approved the final manuscript.
Disclosure statement
Authors declare no actual or potential conflict of interest in relation to this article.
Data availability
Please contact author for data requests.
References
- Quaderni del Ministero della Salute. Criteri di appropriatezza strutturale, tecnologica e clinica nella prevenzione, diagnosi e cura delle patologie andrologiche; 2012; ISSN 2038–5293.
- Valenti D, La Vignera S, Condorelli RA, et al. Follicle-stimulating hormone treatment in normogonadotropic infertile men. Nat Rev Urol. 2013;10:55–62.
- Rago R, Salacone P, Caponecchia L, et al. The semen quality of the mobile phone users. J Endocrinol Invest. 2013;36:970–974.
- Asare-Anane H, Bannison SB, Ofori EK, et al. Tobacco smoking is associated with decreased semen quality. Reprod Health. 2016;13:90.
- Liu Q, Si T, Xu X, et al. Electromagnetic radiation at 900 MHz induces sperm apoptosis through bcl-2, bax and caspase-3 signaling pathways in rats. Reprod Health. 2015;12:65.
- Calogero AE, La Vignera S, Condorelli RA, et al. Environmental car exhaust pollution damages human sperm chromatin and DNA. J Endocrinol Invest. 2011;34:e139–43.
- Italian Parliament. Law 19th February 2004, no. 40: «Norme in materia di procreazione medicalmente assistita». Gazzetta Ufficiale. 24th February 2004, no. 45.
- La Vignera S, Vicari E, Condorelli RA, et al. Male accessory gland infection and sperm parameters (review). Int J Androl. 2011;34:e330–47.
- Attia AM, Abou-Setta AM, Al-Inany HG. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2013;CD005071.
- van Rijkom J, Leufkens H, Crommelin D, et al. Assessment of biotechnology drugs: what are the issues? Health Policy. 1999;47:255–274.
- Zwart-van Rijkom JE, Broekmans FJ, Leufkens HG, From HMG through purified urinary FSH preparations to recombinant FSH: a substitution study. Hum Reprod. 2002;17:857–865.
- Barbonetti A, Calogero AE, Balercia G, et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J Endocrinol Invest. 2018;41:1107–1122.
- La Vignera S, Condorelli RA, Duca Y, et al. FSH therapy for idiopathic male infertility: four schemes are better than one. Aging Male. 2019;1–6. doi:10.1080/13685538.2019.1590696
- Romeo C, Santoro G, Varicocele and infertility: why a prevention? J Endocrinol Invest. 2009;32:559–561.
- Quaderni del Ministero della Salute. Prevenzione, diagnosi e cura delle patologie andrologiche dall’età pediatrica al giovane adulto; 2017; ISSN 2038–5293.
- Condorelli RA, Calogero AE, Mongioi’ L, et al. Varicocele and concomitant dilation of the periprostatic venous plexus: effects on semen viscosity sperm parameters. J Endocrinol Invest. 2016;39:543–547.
- Pallotti F, Paoli D, Carlini T, et al. Varicocele and semen quality: a retrospective case-control study of 4230 patients from a single centre. J Endocrinol Invest. 2018;41:185–192.
- Park YS, Lee SH, Choi HW, et al. Abnormal human sperm parameters contribute to sperm DNA fragmentation in men with varicocele. World J Mens Health. 2018;36:239–247.
- Di Bisceglie C, Fornengo R, Grosso M, et al. Follow-up of varicocele treated with percutaneous retrograde sclerotherapy: technical, clinical and seminal aspects. J Endocrinol Invest. 2003;26:1059–1064.
- Yuan R, Zhuo H, Cao D, et al. Efficacy and safety of varicocelectomies: a meta-analysis. Syst Biol Reprod Med. 2017;63:120–129.
- Esteves SC, Roque M, Agarwal A, Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl. 2016;18:2548.
- Kohn TP, Kohn JR, Pastuszak AW, Varicocelectomy before assisted reproductive technology: are outcomes improved? Fertil Steril. 2017;108:385–391.
- Yilmaz S, Koyuturk M, Kilic G, et al. Effects of leucocytospermia on semen parameters and outcomes of intracytoplasmic sperm injection. Int J Androl. 2005;28:337–342.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103:e18–25.
- Condorelli RA, Russo GI, Calogero AE, et al. Chronic prostatitis and its detrimental impact on sperm parameters: a systematic review and meta-analysis. J Endocrinol Invest. 2017;40:1209–1218.
- Zhao J, Zhang Q, Wang Y, et al. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005.
- Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online. 2015; 30:120–127.
- Galmés Belmonte I. Usefulness and necessity of the andrologist in assisted reproductions units. Actas Urol Esp. 2004;28:364–376.
- Lenzi A, The role of the medical andrologist in the assisted reproduction era. J Endocrinol Invest. 2003;26:268–273.
- Kamischke A, Cordes T, Nieschlag E, The diagnostic of male infertility - an important part of reproductive medicine. Ther Umsch. 2009;66:789–795.