Abstract
Purpose
Prostatic calculi (PCal) are commonly present with prostate disease; we aim to map the incidence and associated clinical risk factors of PCal in Han Chinese.
Material and methods
We retrospectively selected men who sought a medical check-up in 2018. Basic clinical items, including age, weight, height, prostate specific antigen (PSA), uric acid (UA), fasting blood glucose (FBG), urinalysis results, and transabdominal prostate ultrasound, were recorded. Univariate and logistic regression analyses were performed to evaluate whether these factors were associated with the presence of PCal.
Result
We recorded the parameters of laboratory tests and clinical information from 14,427 men; men with PCal comprised 51.65% of the total group and 76.61% of the subgroup of benign prostate hyperplasia (BPH) patients. All the enrolled parameters showed meaningful differences, but the logistic regression analysis only indicated significant effects related to age (OR = 1.044, 95% CI = 1.040–1.047, and p < .001), body mass index (BMI) (OR = 1.035, 95% CI = 1.022–1.048, and p < .001), UA (OR = 0.999, 95% CI = 0.999–1.000, and p = .029), BPH (OR = 2.923, 95% CI = 2.678–3.191, and p < .001), and prostate cysts (OR = 0.609, 95% CI = 0.471–0.788, and p < .001). The odds ratio of the predicted combined model is 1.068.
Conclusions
PCal was detected in 51.65% of men among healthy Han Chinese and in 76.61% of BPH patients. Age, BMI, UA, BPH, and prostate cysts were independent risk factors for the presence of PCal.
Keywords:
Introduction
Prostatic calculi (PCal) are common diseases among men who receive urological outpatient care. There are two general origins of PCal: one form originates in the acini of the prostate due to inflammation [Citation1,Citation2] and the other pathomechanism is the result of chemical prostatitis caused by the intraprostatic reflux of urine [Citation3]. Several parameters, including age, chronic prostatitis (CP), benign prostate hyperplasia (BPH), and alkaptonuria have been reported to be associated with the appearance of PCal [Citation4–7]. The prevalence of PCal is variable; one study based on a Greek population presented a low incidence of approximately 7.4% [Citation8], while Kim et al. [Citation9] determined an incidence of 66.97% in BPH patients in South Korea.
As transabdominal prostate ultrasound and testing for prostate specific antigen (PSA) have become more common in China, the positive detection rate of PCal is increasing rapidly. In this study, to explore the prevalence and clinical risk factors associated with PCal in Han Chinese, we recorded the parameters of laboratory tests and the results of prostate transabdominal ultrasound for men who received health examinations.
Material and methods
Population selection
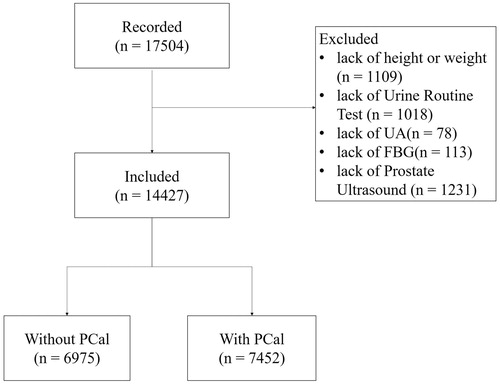
From January 1st to December 31st of 2018, approximately 17,504 men came to the First Affiliated Hospital of Anhui Medical University for routine health examinations and were tested for PSA. The current study was approved by the Institutional Review Board of the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, China, the ethical approval number is Quick-PJ-2019-04-13. In China, PSA and transabdominal prostate ultrasound are commonly performed on men over 50 years old. There are also, however, some younger men who chose to do these examinations as well. Of all the recorded 17,504 men, 3277 were excluded, 1109 of whom lacked data for height or weight, 1018 of whom lacked urinalysis, 78 of whom lacked uric acid (UA) results, 113 of whom lacked fasting blood glucose (FBG) results, and 1231 of whom did not complete a prostate transabdominal ultrasound. The flowchart for population selection is shown in .
Record of clinical characteristics
We extracted and recorded several clinical characteristics for the patients, including age, height, weight, total PSA, UA, FBG, and urinalysis, as well as transabdominal prostate ultrasound results. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). The numbers of red blood cell (RBC) and white blood cell (WBC) per high power field (HPF) were counted by laboratory physicians. Microscopic hamaturia was defined as ≥3 RBC per HPF [Citation10], while pyuria was defined as ria C per HPF [Citation11]. We also divided the participants into 3 age-dependent groups, ≤30 years old, > 30 and ≤50 years old, and > 50 years old, to see the different performance of PCal in different age groups. Meanwhile, according to the BMI criteria of the Asia-Pacific region as defined by World Health Organization (WHO), we stratified these men into 3 groups: group 1 include men who were underweight and normal weight ( < 23 kg/m2), group 2 included overweight men (≥23 and < 25 kg/m2), and group 3 included men who were obese or very obese (≥ 25 kg/m2) [Citation12]. A transabdominal ultrasound examination was used to measure the condition of the prostate by an experienced ultrasound doctor: BPH, PCal, and Prostate cyst were all recorded. We defined PCal as any hyperechoic foci located in the prostate, regardless of its size or location.
Statistics
The distribution of all the clinical characteristics of the enrolled men was displayed with mean, range, or percentage. For the continuous variables, such as age, BMI, PSA, UA, and FBG, we first verified whether the data conformed to normality with a Kolmogorov–Smirnov test, and then determined the difference between groups with and without PCal using student’s t test or Mann–Whitney U test. For the categorical variables, including hematuria, pyuria, BPH, and Prostate cyst, we use Chi-square test for conflicts, as well as for the stratified group regarding age and BMI. Multiple logistical regression analysis was conducted to determine the internal relationship between clinical parameters and the presence of PCal. All the results were calculated using SPSS version 22.0 (SPSS Inc., Chicago, IL,). p < .05 was considered to be statistically significant.
Results
General characteristics of the study
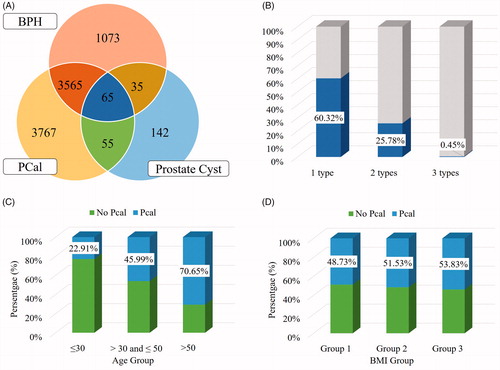
There were 14,427 men who participated in the analysis, of which 6975 men were free of PCal, accounting for approximately 48.35%; PCal was detected in 7452 men (51.65%) (). A total of 8702 (60.32%) men suffered from at least one type of prostate disease and 3720 (25.78%) participants had at least two types of prostate disease, and 65(0.45%) patients had all three pre-set prostate diseases ()).
Correlation analysis
For all the continuous parameters, the results of a Kolmogorov–Smirnov test showed that they did not conform to normality (p < .001), so the Mann–Whitney U test was used to compare each item in the two divided groups. The age of the no PCal group is 41.09 (18–91) years old, significantly less than 51.18 (18–93) in the PCal group (p < .001); the BMI in the PCal group is slightly higher than it in the no PCal group, 24.62(14.20–40.78) vs. 24.38(14.50–41.02) (p < .001); PSA in the PCal group (1.40(0.01–58.99)) is remarkably higher than in the no PCal group(1.02(0.01–39.84), p < .001), which seems to indicate that PCal is associated with an increase of PSA. UA is mildly lower in the PCal group, while FBG is elevated (). For the categorical variables on hematuria and pyuria, the Chi-square test uncovered that the prevalence of hematuria and pyuria in PCal group is elevated when compared to the no PCal group (Phematuria < 0.001 and Ppyuria = 0.033). For the different types of prostate disease detected by ultrasound, BPH showed a positive correlation with PCal (p < .001), while prostate cysts showed a negative link to PCal (p < .001) ().
Table 1. Clinical and demographic features of the patients stratified by PCal.
Prevalence of PCal in different age- and BMI-groups
Because age and BMI were associated with the appearance of PCal, we divided the participants into different groups based on age and BMI to assess prevalence in these groups. As to age groups, the prevalence of PCal was only 22.91% in men under 30 years old; in the group > 30 and ≤50 years old group, 45.99% had PCal; and the highest prevalence of PCal was in the group over 50, accounting for approximately 70.65%. The prevalence of PCal was higher in the older groups, and this difference was statistically significant (p < .001) (). The rates of PCal in men in the BMI based groups showed the same results as with the age-based groups: the prevalence of PCal increased from group 1–3. In group 1, with a BMI less than 23 kg/m2, approximately 48.73% had PCal. In group 2, the rate closes to 51.53%, while the prevalence of PCal in obese and very obese men was 53.83% ().
Logistic regression analysis
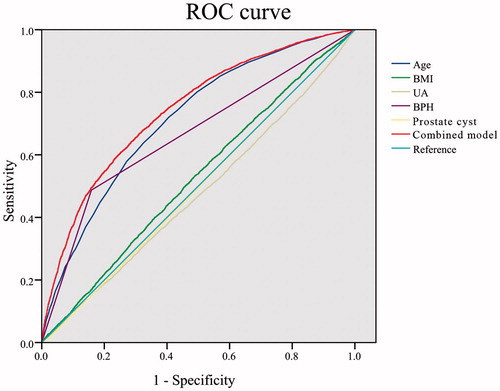
To exclude the internal effects of the participants, such as age or BMI difference between the PCal group and the no PCal group, we carried out a logistic regression analysis between all the recorded clinical parameters and PCal status. Using the “Forward: Conditional” method of logistic regression analysis, age, BMI, UA, BPH, and prostate cyst were enrolled in the model generated at the last step, while other parameters were excluded, including PSA, FBG, hematuria, and pyuria. The OR and 95% CI of each item are shown in . We also used the receiver operating characteristic (ROC) curve to display the prediction efficiency of the combined model and each retained item (). The area under curve (AUC) value of the combined model is 0.742 ().
Table 2. Multivariate logistic regression analysis of factors effective on PCal.
Discussion
PCal is one of the most prevalent prostate diseases among males. Some studies claim that PCal occurs physiologically with normal aging and has little significance [Citation13,Citation14], while others claim it is associated with CP, BPH, or prostate cancer. The incidence of PCal varies around the world, perhaps due to dietary patterns, different definitions, populations, or detection methods. With the help of transrectal ultrasonography (TRUS), the prevalence of PCal in South Korea was determined to be between 36.1 and 76.6% [Citation15,Citation16]. Another study conducted in Italy uncovered an incidence of 25.3% through TRUS or prostate biopsy [Citation17]. One earlier autopsy study claimed that the incidence of PCal was 70.1 and 29.1% in Black men from Washington, DC, and from Ibadan, Nigeria and Accra, and Ghana, respectively, which suggest that dietary patterns affect the prevalence of PCal.
The incidence rates of PCal in China are not consistently reported in different studies. Fei et al. [Citation18] reported an incidence of 43.7% in chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients at northeast China. Zhao et al. [Citation19] indicated a 38.7% incidence in east China, based on a sample of 106 men who were diagnosed with chronic bacteria prostatitis. Wang and Li [Citation20] determined the prevalence of PCal in BPH patients as approximately 89.54% in southeast China. More recently, a study based on routine check-ups of men in southeast China demonstrated a lower incidence of 20.1%. In our study, we initially enrolled 17,504 men who presented for routine health examinations, and, after excluding some patients who lacked necessary data, analyzed the data for 14,427 patients. The prevalence of PCal as shown in this study was approximately 51.65%, and among men with BPH, the incidence of PCal increased to 76.61%.
Age is one of the important factors in both the prevalence and the burden of PCal [Citation8,Citation16]. In this study, we first found the relationship between age and PCal with the Mann–Whitney U test; the average age of people with PCal is significantly high than that of people without PCal. Meanwhile, for the subgroup analysis using age groups, the prevalence of PCal in men > 50 years old is as high as 70.65%, while in men e30 years old is only 22.91%. PSA is always associated with the appearance of CP, BPH, or prostate cancer [Citation21,Citation22], while, as mentioned above, PCal is often detected along with these three prostate diseases. Thus, we assessed whether PSA is linked to PCal, as well as to the other PSA related clinical parameters, BMI, and FBG [Citation23–26]. As the results show, there are remarkable differences between men with PCal and men without PCal for each parameter in the single factor analysis, but the results of multiple logistic regression show that PSA and FBG are excluded after adjustment of other risk factors, and only BMI still affects the presence of PCal.
The status of inflammation and symptom duration of CP are also linked to PCal in men, but the interrelation and mechanism are still not clear [Citation27]. Some studies have reported that PCal is seen in patients with recurrent bacterial urinary infection, which may be another cause of calculi [Citation19]. In our study, each patient underwent urinalysis, so we recorded the presence of hematuria and pyuria to indicate urinary infection. Single factor analysis shows a slight evaluation of hematuria and pyuria in PCal men, however, these differences were eliminated by other factors in the logistic regression analysis. Benign prostatic hyperplasia (BPH) is another possible factor affecting PCal, because the enlarged gland mass might compress the prostate ducts [Citation28,Citation29]. BPH and prostate cysts are all statistically associated with PCal in both single factor analysis and logistic regression analysis. In all the 14,427 men analyzed, about half also suffered from BPH in the PCal group, a much larger proportion than in the no PCal group. In contrast, prostate cysts might represent a protective factor for PCal, though the underlying mechanism awaits further research. Alkaptonuria, UA, and calcium oxalate are also reported to lead to the formation of PCal [Citation30–32]. Balasar et al. [Citation32] reported that UA is higher in PCal men and associated with the formation of PCal based on a sample of 169 men. Our study also found a large interrelation between UA and PCal, with the average UA level in the PCal group being lower than in the no PCal group, though the high UA could be related to low serum calcium [Citation33].
This study evaluated the prevalence and clinical risk factors associated with PCal. Univariate analysis and logistic regression analysis proved that age, BMI, UA, BPH, and prostate cysts are risk factors for PCal. Nevertheless, there are some limitations of the study that cannot be neglected. First, we did not obtain the disease history of all the men, especially the history of CP and any related international prostate symptom score (IPSS) or National Institutes of Health Chronic Prostatitis Symptom Index (NHI-CPSI) scores, and thus could not evaluate how CP might affect the formation of PCal. Second, all the clinical parameters are only single records and this might cause a random bias, but the large number of 14,427 samples is a good way to decrease the bias. Third, all the enrolled men were men who presented voluntarily for a check-up, and due to the high cost of a check-up there could be selection bias towards men who live in urban areas, and against those with lower income. Therefore, all the results should be interpreted with caution.
Conclusions
The prevalence of PCal in Han Chinese is 51.65% and increases to 76.61% in BPH patients. Age, BMI, UA, BPH, and prostate cysts are independent risk factors for the presence of PCal.
Acknowledgements
The authors are very thankful to Ms. Huiya Ma for the working on data extraction.
Disclosure statement
The authors have declared that no conflict of interests exists.
Additional information
Funding
References
- Sfanos KS, Wilson BA, De Marzo AM, et al. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA. 2009;106:3443–3448.
- De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269.
- Kirby RS, Lowe D, Bultitude MI, et al. Intra-prostatic urinary reflux: an aetiological factor in abacterial prostatitis. Br J Urol. 1982;54:729–731.
- Dessombz A, Meria P, Bazin D, et al. Prostatic stones: evidence of a specific chemistry related to infection and presence of bacterial imprints. PLoS One. 2012;7:e51691.
- Zhao Z, Xuan X, Zhang J, et al. A prospective study on association of prostatic calcifications with sexual dysfunction in men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). J Sex Med. 2014;11:2528–2536.
- Masoud HMF, Alhawari HH, Alryalat NT, et al. A rare presentation of alkaptonuria: extensive prostatic calculi with highlight of stones found in a unique paraprostatic urethral diverticulum. Int J Surg Case Rep. 2017;38:192–195.
- Guo W, Chen M, He X, et al. Component analysis of prostatic calculi and the causes of calculus formation. Biomed Res. 2016;4:1223–1227.
- Geramoutsos I, Gyftopoulos K, Perimenis P, et al. Clinical correlation of prostatic lithiasis with chronic pelvic pain syndromes in young adults. Eur Urol. 2004;45:333–337.
- Kim SJ, Lee BH, Lee BY, et al. Relations between prostatic calculi and lower urinary tract symptoms of benign prostatic hyperplasia. J Korean Continence Soc. 2009;13:30–36.
- Davis R, Jones JS, Barocas DA, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188:2473–2481.
- Abrahamian FM, Krishnadasan A, Mower WR, et al. Association of pyuria and clinical characteristics with the presence of urinary tract infection among patients with acute nephrolithiasis. Ann Emerg Med. 2013;62:526–533.
- Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163.
- Leader AJ, Queen DM. Prostatic calculous disease. J Urol. 1958;80:142–146.
- Sondergaard G, Vetner M, Christensen PO. Prostatic calculi. Acta Pathol Microbiol Immunol Scand A. 1987;95:141–145.
- Hong CG, Yoon BI, Choe HS, et al. The prevalence and characteristic differences in prostatic calcification between Health Promotion Center and urology department outpatients. Korean J Urol. 2012;53:330–334.
- Park B, Choo SH. The burden of prostatic calculi is more important than the presence. Asian J Androl. 2017;19:482–485.
- Dell’Atti L, Galosi AB, Ippolito C. Prostatic calculi detected in peripheral zone of the gland during a transrectal ultrasound biopsy can be significant predictors of prostate cancer. Arch Ital Urol Androl. 2016;88:304–307.
- Fei X, Jin W, Hua S, et al. Prospective study on association of prostatic calcifications with clinical symptoms and results of treatment in men with type III prostatitis. Sci Rep. 2017;7:5234.
- Zhao WP, Li YT, Chen J, et al. Prostatic calculi influence the antimicrobial efficacy in men with chronic bacterial prostatitis. Asian J Androl. 2012;14:715–719.
- Wang YX, Li MQ. Correlation between prostatic calculi and prostate volume of patients with benign prostatic hyperplasia. J Mod Urol. 2014;19:800–802.
- Xia SJ, Cui D, Jiang Q. An overview of prostate diseases and their characteristics specific to Asian men. Asian J Androl. 2012;14:458–464.
- Irani J, Millet C, Levillain P, et al. Serum-to-urinary prostate-specific antigen ratio: a potential means of distinguishing benign prostatic hyperplasia from prostate cancer. Eur Urol. 1996;29:407–412.
- Yue L, Ge Y, Wang T, et al. The correlation between body mass index and prostatic-related parameters in men 40 years or older in Zhengzhou. Aging Male. 2018;17:1–6.
- Aref AT, Vincent AD, O’Callaghan ME, et al. The inverse relationship between prostate specific antigen (PSA) and obesity. Endocr Relat Cancer. 2018;25:933–941.
- Sarma AV, Hotaling J, Dunn RL, et al. Poor glycemic control is associated with reduced prostate specific antigen concentrations in men with type 1 diabetes. J Urol. 2015;193:786–793.
- Han JH, Lee YT, Kwak KW, et al. Relationship between insulin resistance, obesity and serum prostate-specific antigen levels in healthy men. Asian J Androl. 2010;12:400–404.
- Shoskes DA, Lee CT, Murphy D, et al. Incidence and significance of prostatic stones in men with chronic prostatitis/chronic pelvic pain syndrome. Urology. 2007;70:235–238.
- Engelhardt PF, Seklehner S, Brustmann H, et al. Tumor necrosis factor-α expression in patients with obstructive benign prostatic hyperplasia is associated with a higher incidence of asymptomatic inflammatory prostatitis NIH category IV and prostatic calcification . Scand J Urol. 2015;49:472–478.
- Klimas R, Bennett B, Gardner WA Jr. Prostatic calculi: a review. Prostate. 1985;7:91–96.
- Sun C, Xie G, Huang F, et al. Effects of calcium oxalate on expression of clusterin and lower urinary tract symptoms in prostatitis and benign prostatic hyperplasia patients with calculi. Med Sci Monit. 2018;24:9196–9203.
- Sali G, Thomas A, Kumar G, et al. Extensive prostatic calculi in alkaptonuria: an unusual manifestation of rare disease. Asian J Urol. 2015;2:179–181.
- Balasar M, Sonmez MG, Aydin A, et al. Is there a relation between serum uric acid values and prostatic calculi presence? Urol Int. 2019;102:199–204.
- Kuroczycka-Saniutycz E, Porowski T, Protas PT, et al. Does obesity or hyperuricemia influence lithogenic risk profile in children with urolithiasis? Pediatr Nephrol. 2015;30:797–803.