Abstract
Objectives
To define if less number of cores would be sufficient to diagnose prostate cancer (PCa) in men with PSA levels >20 ng/ml and to reveal the cancer detection rates in this population.
Methods
The data of the men who had 12-core prostate biopsy with a PSA value >20 ng/mg were reviewed. We recorded age, prostate volume, PSA level, and pathology report findings. Patients grouped according to PSA levels and compared for PCa detection rates, and several parameters. We created 16 prostate biopsy scenarios (S1–S16) and applied these to our database to find out the best biopsy protocol to detect PCa.
Results
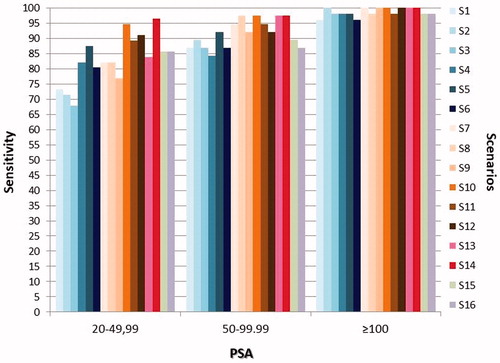
A total of 336 patients with a mean age of 70.5 (47–91) years were included. Mean PSA level was 190.6 (20–5474) ng/ml. PCa detection rates were 55.3%, 81.0%, and 97.7% in patients with PSA levels 20–49.99, 50–99.99, and ≥100 ng/ml, respectively. PSA level was correlated to clinically more important digital rectal examination findings. We selected 2 cores in S1–S6, 4 cores in S7–S12, and 6 cores in S13–S16. We calculated the sensitivity of each scenario and found that all scenarios in PSA Group 3 had a sensitivity >95%. In Group 2, S8, S10, S13, and S14 and in Group 1, only S14 had sensitivity >95%.
Conclusions
It is not necessary to take 10–12 core biopsy samples in men with PSA levels >20 ng/ml. We recommend taking 2, 4, and 6 samples for patients with PSA levels ≥100 ng/ml, 50–99.99 ng/ml, and 20–49.99 ng/ml, respectively.
Introduction
Prostate cancer (PCa) has been one of the leading healthcare problems worldwide, as it is the second most common cancer and the fifth most common cancer-related mortality in men [Citation1]. In developed countries, PCa is the most incidental cancer and third in mortality [Citation2]. Although the introduction of prostate-specific antigen (PSA) has resulted in an increase in the incidence of prostate cancer because of extensive screening programs worldwide, it also led to controversies and questions. As a diagnostic test which is also used for screening, the first issue was to determine a cutoff value and a reference range of 0 to 4 ng/ml was defined as normal PSA level. This cutoff was further confirmed by Catalona et al. for performing prostate biopsies [Citation3]. Later on, several PSA derivatives such as PSA density, PSA velocity and doubling time, free/total PSA ratio, and more recently, advanced tests such as Prostate Health Index (PHI), prostate cancer gene 3 (PCA3) were used to determine the risk of having PCa and if there was a need to take prostate biopsy.
The reason for developing these noninvasive tests and not proceeding directly with prostate biopsy in case of an elevated PSA or suspicious test results was that prostate biopsy was not without complications. Previous studies have shown that nearly 60% of the patients having prostate biopsies had complications [Citation4,Citation5]. These include urinary tract infections, fever, and hospitalization with an incidence of about 4% and transient hematuria, hematospermia, and rectal bleeding in more than 80% of the patients [Citation6,Citation7].
Another controversy about PCa diagnosis after PSA era and defining of PSA cutoffs was the optimal number of cores or samples during prostate biopsies. The systematic sextant biopsy protocol which was introduced by Hodge et al. [Citation8] was used as the gold standard until late 1990s however it was later demonstrated that this protocol could miss cancers located in different locations of the prostate [Citation9]. Sextant biopsy is no longer considered adequate and current guidelines and expert panels recommend initial prostate biopsy protocols involving 10–12 cores in men with an abnormal digital rectal examination finding or a high PSA level [Citation10–12].
Increasing the number of cores in prostate biopsy decreases the probability of missing PCa diagnosis however has the potential risk of increasing the rate of complications including pain, hematuria, hematospermia, urethrorrhagia, rectal bleeding, and infection ranging from a simple urinary tract infection to sepsis [Citation13]. Previous studies have reported that when the number of cores increased, significantly greater rectal bleeding and hematospermia were observed [Citation14,Citation15]. One overlooked fact is that a significant rate of older man having prostate biopsies has a tendency to bleeding because of anticoagulant or antiplatelet drugs. In these men, bleeding and other complications could become life threatening. Moreover, most patients are aware that the process is painful and their anxiety is likely to increase once they are informed about the complications. To avoid these, it is important to determine and if necessary, limit the optimal number of biopsy sites for each patient, taking into account age, PSA level, digital rectal examination (DRE) findings, and prostate volume (PV) [Citation5,Citation13].
In the literature, studies regarding PSA thresholds and the optimal number of cores during prostate biopsies were mainly focused on the patient groups who had PSA levels between 2.5 and 10 ng/ml or PSA levels under 20 ng/ml. Most of the clinicians and patients think that once they had a PSA level of >20 ng/ml, there would be a high probability of having PCa. In the literature and clinical guidelines however, there is a paucity of data for the exact cancer detection rates which would help significantly in counseling these patients [Citation10,Citation16].
Although there are a few studies showing that less than 10 or 12 cores would be enough to diagnose PCa in men with elevated PSA levels, these were not individualized for different PSA cutoff levels. Therefore, in this study we aimed to define if less number of cores would be sufficient to diagnose PCa in men with PSA levels >20 ng/ml and also if the number of cores would be individualized for different PSA cutoff levels. We also aimed to reveal the cancer detection rates in men with PSA levels >20 ng/ml in our region to compare with the other populations and to counsel patients better.
Patients and methods
After the approval of our institutional ethics committee, the data of the men who had transrectal ultrasound (TRUS) guided 12-core prostate biopsy with a PSA value >20 ng/mg between 2010 and 2017 were retrospectively reviewed. We recorded age, prostate volume, total and free PSA levels, pathology report (final diagnosis, the number of positive cores, and Gleason scores if the diagnosis was cancer). Patients were grouped according to their PSA levels; 1st group: 20–49.99 ng/ml, 2nd group: 50–99.99 ng/ml, and 3rd group: ≥100 ng/ml. These groups were compared for PCa detection rates, Gleason scores, the number of positive cores, and perineural invasion (PNI).
We created 16 prostate biopsy scenarios () and applied these to our database as if we did not know the final pathology results. We tried to find out the best biopsy protocol in each group to detect PCa accurately.
Table 1. Different scenarios (hypothesis) to define the optimal number of cores in prostate biopsies.
TRUS guided biopsy protocol
Patients were placed in the lateral decubitus position with the right knee flexed towards the belly, and TRUS guided needle biopsy was performed. A standard 12-core biopsy protocol (6 laterally targeted biopsies in addition to para-sagittal sextant biopsies) was used for each patient. An ultrasound with a 7.5 MHz transrectal probe (BK Medical) and an automatic biopsy instrument attached to an 18-gauge needle was used. Transverse and longitudinal section images were obtained and prostate volume was measured before taking the samples. The prostate volume was calculated by applying the prostate ellipsoid formula (prostate size = 0.5233 × length × width × anteroposterior length). A 19 G Chiba needle and 10 ml, 2% prilocaine was used for periprostatic block local anesthesia and a 20 cm, 18 G automatic biopsy needle were used to perform biopsies. Prior to the procedure, urinary tract infection was ruled out with urinalysis and urine culture. Antibiotic prophylaxis was given to all patients.
Statistical analysis
The data analysis was performed using SPSS (Statistical Package for Social Sciences) for Windows Version 21.0 (SPSS Inc, Chicago, Illinois, USA). Descriptive statistics for variables with a normal distribution and categorical variables were shown as mean ± standard deviation and percentage. Chi-square test was used for the analysis of continuous variables and one way ANOVA test and Kruskal Wallis tests were used to compare continuous variables. Any p values <.05 was considered as significant.
Before we set up the scenarios that we have mentioned above, we tried to find a way to select the most appropriate sample number and site(s). After a few discussions with the scientists in the Department of Engineering, we decided to use an artificial intelligence technique that could also “learn” from an existing database. For that purpose, we chose support vector machines which were supervised learning models with associated learning algorithms that analyze data used for classification and regression analysis. When it is not possible to classify data in a linear method, a nonlinear classification is used as in our study. We used support vector machines (SVM) to define if any of the cores taken during standard prostate biopsy contributed to the results more than the others.
Regarding biopsy scenarios, a sensitivity of >95% was considered as significant.
Results
A total of 336 patients with a mean age of 70.5 (47–91) years were included in our study. Mean PSA value was 190.6 (20–5474) ng/ml. PCa detection rates were 55.3%, 81.0%, and 97.7% in groups 1, 2, and 3, respectively (p < .0001). In further analysis of patients diagnosed as PCa, pathological examination revealed a Gleason score >7 in 41.4%, 63%, and 78.8% of the patients in groups 1, 2, and 3, respectively (p < .001) ().
Table 2. Pathological diagnosis in different PSA levels.
We showed that, a higher PSA level was associated with higher Gleason scores and PNI (). When patients were grouped according to prostate volume (<50 cc and ≥50 cc), there was a higher probability of having prostate cancer (80.1%) in patients with a prostate volume <50 cc when compared to the group having a prostate volume ≥50 cc (65.3%), (p = .02). When we further analyzed this relation in PSA groups separately, a prostate volume <50 cc was significantly related to having PCa diagnosis in groups 1 and 2 (PSA < 100 ng/ml) (p = .04), however prostate volume was not correlated with PCa diagnosis in group 3 (PSA ≥ 100 ng/ml).
Table 3. Perineural invasion in different PSA levels and Gleason scores.
We classified the DRE findings as soft-benign, rigid and invasive, and found that a higher PSA level was correlated to clinically more important DRE findings (rigid and/or invasive) (). In patients with PSA levels 20–99.99 ng/ml positive DRE findings were significantly associated with PCa diagnosis. In patients without any nodules in DRE, a higher PSA level was related to have PCa diagnosis, however in patients having nodules palpated there was no correlation between PSA level and PCa diagnosis ().
Table 4. Prostate cancer in different PSA levels according to digital rectal examination findings.
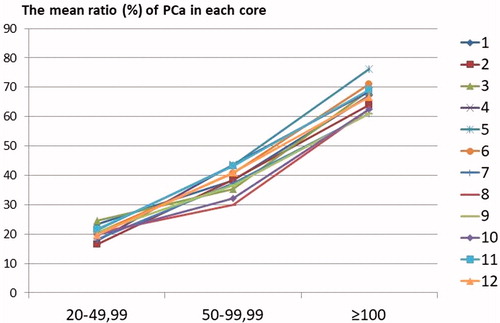
We calculated the mean ratio (%) of PCa in the samples taken from each core. The highest cancer ratio was found in right base para-sagittal samples in the third group (PSA ≥100 ng/ml) as 76.3% and the lowest was found in right apex lateral in the first group (PSA level 20–49.99 ng/ml) as 16.6%. We showed that the cancer ratio in tissue samples increased with an increase in the PSA level ().
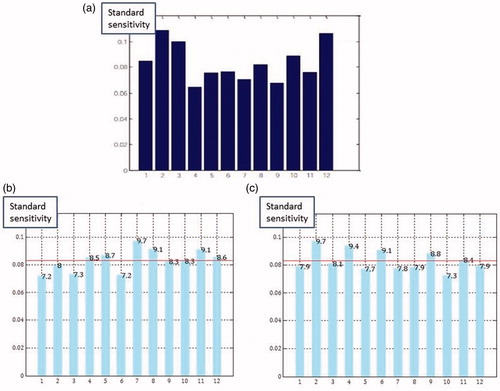
Since our aim was to detect the minimal number of cores needed to diagnose PCa, we tried to figure out if any of the cores were significantly contributing to the results. We uploaded all the data to a computer having a software using SMV and let the computer to define if any of the 12 cores were dominating the outcomes. We did the same process for all 3 PSA groups and observed that none of the cores were significantly dominating the biopsy outcomes (). After we have found that none of the cores were leading PCa diagnosis, we designated 16 different biopsy scheme scenarios (S1–S16) and tried to find out what would be the outcome if we had taken biopsies using these scenarios.
Figure 2. The results of analysis of support vector machines for PSA levels 20–49.99 ng/ml (a), 50–99.99 ng/ml (b), and ≥100 ng/ml (c).
We selected 2 cores in S1–S6, 4 cores in S7–S12, and 6 cores in S13–S16. We calculated the sensitivity of each scenario () and found that all of the scenarios in PSA Group 3 had a sensitivity >95%. In Group 2, S8, S10, S13 and S14, and in Group 1, only S14 had sensitivity >95%.
Discussion
The majority of the studies in the literature about prostate cancer screening and diagnosis were mainly focused on the patients with a PSA <20 ng/ml. Despite recent advances in technology, biomarkers, and screening, a TRUS guided systematic prostate biopsy is required to obtain tissue for the histopathological diagnosis of prostate cancer. Current practice, as recommended by guidelines is to take 10–12 core systematic biopsies for the diagnosis of prostate cancer. Therefore, most urology departments are offering men attending the outpatient or prostate clinics extended and multiple biopsy protocols in men with a PSA level suspicious of PCa, independent of the specific PSA level for each individual. In this study, we demonstrated that it is unnecessary to perform 12 core needle biopsy sampling of the prostate in men with a PSA level >20 mg ml and it would be enough to take 2, 4, and 6 samples for patients with PSA levels of ≥100 ng/ml, 50–99.99 ng/ml, and 20–49.99 ng/ml, respectively. There are few studies investigating the optimal number of cores in the literature. In their retrospective analysis of 436 patients with a PSA >10 ng/ml, Philip et al. [Citation5] showed that TRUS guided prostate biopsy required only 8 cores in patients with PSA 10–20 ng/ml and 6 cores concentrated on the apical region would be sufficient in men with PSA >20 ng/ml to diagnose PCa.
In this study, a prospective database was used for the analysis and for the first time a software using SVM which was a kind of artificial intelligence was used to detect if any of the 12 cores were dominating the outcomes. If we had a dominant core or cores after that analysis, we would use these for further analysis and we also would recommend taking biopsies from these cores initially. However, we could not find any dominant cores so we decided to create different scenarios as if we were performing the biopsies for the first time, without knowing the outcomes. Of course, the number of scenarios can be increased to hundreds however we limited the number to 16 choosing the most convenient and possible alternatives.
We did not include the complications of TRUS guided prostate biopsies in this report because it was beyond the aims of our study. Significant complications such as fever, sepsis, hematuria, and rectal bleeding can be seen after prostate biopsy. Eichler et al. [Citation11] reported 80–82% hematuria and hematospermia rates and they also showed that complication rates were decreased with a reduced number of cores. Ghani et al. [Citation14] also reported that rectal bleeding decreased with the reduction of the number of cores taken during biopsy. Therefore, our protocol of reducing the number of cores according to PSA thresholds would help decreasing the complications without compromising the accuracy of prostate biopsies.
The overall PCa detection rate in our study was 72.3%. As expected, there was a proportional increase in cancer detection rates with higher PSA levels which were 55.3%, 81.0%, and 97.7% in patients with PSA levels of 20–49.99 ng/ml, 50–99.99 ng/ml, and ≥100 ng/ml, respectively. There are few studies reporting the incidence of PCa in patients with high PSA levels. Philip et al. [Citation5] found a 43% overall cancer detection rate in men with PSA levels >10 ng/ml. However, 56% of the patients in this study had PSA levels of 10.1–20 ng/ml and cancer detection in patients with PSA levels >20 ng/ml was 82.3%. In another study from United States, a cancer detection rate of 87% at a PSA cut off level of 20 ng/ml [Citation17]. Yang et al. have found a PCa detection rate of 66.2% in Korean men [Citation18]. Our PCa detection rate was somewhere between these reports, therefore, we can conclude that PCa incidence in high PSA levels is correlated to PCa incidence in the country. Since the majority of the studies in the literature have focused on the cancer detection rates and biopsy protocols in men with PSA levels <10 ng/ml or <20 ng/ml, we believe that our study would contribute to the epidemiology of PCa and help counseling patients better.
In our study, mean patient age is high since we had included only the patients with PSA levels >20 ng/ml. American Urological Association (AUA) does not recommend routine PSA screening for men 70 or older or with a life expectancy of less than 10–15 years. However, our health policy does not have such a restriction and since we are a referral center, we have admissions and referrals from primary and secondary care centers with an already performed PSA test frequently. In the study of Yang et al. [Citation18] the mean age of the study population was 69.7 years (range: 38–91), similar to our study. We think that geographical differences and government health policies might have a role in that.
PNI is defined as the presence of cancer cells tracking along or around a nerve within the perineural space and it has been demonstrated that PNI may be a route of metastasis for many different cancers. PNI has been widely investigated as a prognostic marker for PCa and a recent systematic review and meta-analysis suggested that the presence of PNI was associated with higher risk of biochemical recurrence PCa following radical prostatectomy or radiotherapy [Citation19]. PNI was also associated with upgrading of the Gleason Scores that had been reported in prostate biopsies after pathological examination of the final surgical specimen [Citation20,Citation21]. In their series of 1550 patients with localized PCa, Lee et al. found that there was a significant association between the presence of PNI and adverse pretreatment risk-features, including higher clinical T-stage, higher biopsy Gleason score, and higher mean PSA [Citation21]. Similarly, Vukovic et al. reported a strong positive correlation of PNI with clinical stage and patient age, Additionally, they demonstrated that PNI was a non-independent risk factor for metastatic occurrence and biochemical cancer recurrence. However, they could not find a correlation between PNI and Gleason score or PSA [Citation22]. Although investigated as a prognostic marker previously, to our knowledge, PNI and PSA correlation was not investigated before in patients with high PSA levels. In our study, higher Gleason scores and more PNI were found with increasing PSA levels.
Abnormal DRE findings are among the indications of prostate biopsy and also show the extent of the disease clinically. Yang et al. [Citation18] found that, in patients with positive DREs, PCa detection rates were 24.1%, 53.9%, 66.3%, and 94.1% in patients with PSA levels 4–10 ng/ml, 10–20 ng/ml, 20–100 ng/ml, and >100 ng/ml, respectively whereas these rates were 11.8%, 16.8%, 64.9%, and 90.1% in patients with negative DREs. In this study, in patients with PSA levels between 4 and 20 ng/ml, significant differences in the detection rate were found according to DRE findings. Similarly, in the current study, we have found that a higher PSA level was correlated to clinically more important DRE findings and in patients with PSA levels, 20–99.99 ng/ml positive DRE findings were significantly associated with PCa diagnosis. Moreover, in patients without nodules in DRE, there was no correlation between PSA level and PCa diagnosis, however in patients without any nodules in DRE, a higher PSA level was related to PCa diagnosis.
The probability of finding cancer cells during prostate biopsy increases with a decrease in prostate volume in a standard 10–12 core TRUS guided prostate biopsy [Citation13]. In our study, prostate volume <50 cc was related to having PCa diagnosis in patients with PSA < 100 ng/ml however prostate volume was not correlated with PCa diagnosis when PSA was ≥100 ng/ml. Percentage of cancer volume in prostate biopsy cores was investigated and found as a prognostic factor in previous studies [Citation23–25]. In accordance, we showed that the cancer ratio in tissue samples increased with an increase in the PSA level.
In conclusion, patients with PSA levels >20 ng/ml has high cancer detection rates. In our series, we found a 72.3% overall PCa detection rate which increased with higher PSA levels. Unlike current guidelines and recommendations, it is not necessary to take 10–12 core biopsy samples in men with PSA levels >20 ng/ml. We demonstrated that it would be enough to take 2 samples from patients with PSA levels of ≥100 ng/ml, and 4 and 6 samples for patients with PSA levels of 50–99.99 ng/ml, and 20–49.99 ng/ml, respectively. We believe that it would be possible to decrease biopsy-related complications with the widespread use of limited biopsy protocols in patients with high PSA levels in the future.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Na R, Ye D, Qi J, et al. Prostate health index significantly reduced unnecessary prostate biopsies in patients with PSA 2-10 ng/mL and PSA >10 ng/mL: results from a Multicenter Study in China. Prostate. 2017;77:1221–1229.
- Torrealba N, Vera R, Fraile B, et al. TGF-beta/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male. 2019;1-11. DOI:10.1080/13685538.2019.1597840
- Catalona WJ, Hudson MA, Scardino PT, et al. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2037–2042.
- Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166:856–860.
- Philip J, Manikandan R, Javle P, et al. Prostate cancer diagnosis: should patients with prostate specific antigen >10ng/mL have stratified prostate biopsy protocols? Cancer Detect Prev. 2009;32:314–318.
- Loeb S, van den Heuvel S, Zhu X, et al. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–1114.
- Ragavan N, Philip J, Balasubramanian SP, et al. A randomized, controlled trial comparing lidocaine periprostatic nerve block, diclofenac suppository and both for transrectal ultrasound guided biopsy of prostate. J Urol. 2005;174:510–513. discussion 513.
- Hodge KK, McNeal JE, Stamey TA. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142:66–70.
- Cormio L, Scattoni V, Lorusso F, et al. Prostate cancer detection rates in different biopsy schemes. Which cores for which patients? World J Urol. 2014;32:341–346.
- EAU Guidelines. Edn. presented at the. ISBN 978-94-92671-01-1. EAU Annual Congress Copenhagen; 2018. Available from: www.uroweb.com
- Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–1612.
- Shariat SF, Roehrborn CG. Using biopsy to detect prostate cancer. Rev Urol. 2008;10:262–280.
- Kocan H. Factors affecting the diagnosis of prostate cancer through 12 quadrant guided prostate biopsy. Aging Male. 2019;1–6. DOI:10.1080/13685538.2019.1573219
- Ghani KR, Dundas D, Patel U. Bleeding after transrectal ultrasonography-guided prostate biopsy: a study of 7-day morbidity after a six-, eight- and 12-core biopsy protocol. BJU Int. 2004;94:1014–1020.
- Naughton CK, Miller DC, Mager DE, et al. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164:388–392.
- Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. American Urological Association (AUA) Guideline; 2018. Available from: www.auanet.org
- Gerstenbluth RE, Seftel AD, Hampel N, et al. The accuracy of the increased prostate specific antigen level (greater than or equal to 20 ng./ml.) in predicting prostate cancer: is biopsy always required? J Urol. 2002;168:1990–1993.
- Yang WJ, Lee DH, Chung BH, et al. Detection rate of prostate cancer on biopsy according to serum prostate-specific antigen in Korean men: a multicenter study. Urology. 2006;67:333–336.
- Zhang LJ, Wu B, Zha ZL, et al. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiotherapy: a systematic review and meta-analysis. BMC Urol. 2018;18:5.
- Athanazio D, Gotto G, Shea-Budgell M, et al. Global Gleason grade groups in prostate cancer: concordance of biopsy and radical prostatectomy grades and predictors of upgrade and downgrade. Histopathology. 2017;70:1098–1106.
- Lee IH, Roberts R, Shah RB, et al. Perineural invasion is a marker for pathologically advanced disease in localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1059–1064.
- Vukovic M, Kavaric P, Magdelinic A, et al. Perineural invasion on biopsy specimen as predictor of tumor progression in aging male treated with radical prostatectomy. Could we use it for pre-surgical screening? Aging Male. 2019;1–6. DOI:10.1080/13685538.2019.1581758
- Erdogan A, Polat S, Keskin E, et al. Is prostate volume better than PSA density and free/total PSA ratio in predicting prostate cancer in patients with PSA 2.5-10 ng/mL and 10.1-30 ng/mL? Aging Male. 2019;1–7. DOI:10.1080/13685538.2019.1578741
- Slater JM, Bush DA, Grove R, et al. The prognostic value of percentage of positive biopsy cores, percentage of cancer volume, and maximum involvement of biopsy cores in prostate cancer patients receiving proton and photon beam therapy. Technol Cancer Res Treat. 2014;13:227–231.
- Vance SM, Stenmark MH, Blas K, et al. Percentage of cancer volume in biopsy cores is prognostic for prostate cancer death and overall survival in patients treated with dose-escalated external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:940–946.