Abstract
Aim: To investigate the relationship between sarcoidosis and metabolic syndrome (MetS) and insulin resistance (IR).
Method: In our study, 47 patients with sarcoidosis who applied to our outpatient clinic and 45 healthy individuals without chronic disease were included. All patients were evaluated for MetS according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATP III) criteria. The presence of three of the five factors defined by ATP III for MetS was accepted as a diagnosis of MetS. IR is calculated using the HOMA-IR index.
Results: The mean age of the 47 patients with sarcoidosis was 50.7 ± 12.2 years and the mean age of the 45 control groups was 42.9 ± 14.4 years. Almost 80% of the patients were diagnosed as stage 2 sarcoidosis. Distribution of the patients according to the use of steroid is; almost half of the patients (47%) received steroid previously or recently. Patients with sarcoidosis have a 7.66 relative risk for MetS, whereas they also have a 5.48 relative risk of insulin resistance development.
Conclusion: This study shows that MetS is associated with increased sarcoidosis risk. MetS and IR diagnosis was higher in patients with sarcoidosis.
Introduction
Sarcoidosis is a chronic systemic disorder characterized by granulomatous inflammation in response to an unidentified antigen [Citation1]. It shows a consistent predilection for adults. Significant diversity in prevalence, disease presentation, and severity occur among different ethnic and racial groups. The diagnosis of sarcoidosis needs an appropriately clinical picture, histological demonstration of non-caseating granulomas, and excluding of other diseases capable of producing a correlative histological or clinical picture [Citation2]. Sarcoidosis presenting features are protean, ranging from asymptomatic but abnormal findings on chest radiography in many patients to progressive multiorgan failure in an unfortunate minority [Citation3].
Metabolic syndrome (MetS) is a multifactorial pathological condition characterized by the presence of any three of the following five risk factors: hypertension, hyperglycemia, hypertriglyceridemia, low level of high-density lipoprotein (HDL)-cholesterol, and an enlarged waist circumference [Citation4]. Obesity increases the risk of morbidity of cardiovascular disease, type 2 diabetes mellitus, dyslipidemia, stroke, osteoarthritis, gallbladder disease, sleep apnea, respiratory problems, and some cancers [Citation5]. Obesity has also been associated with increased risk of different autoimmune diseases including psoriasis, rheumatoid arthritis, and inflammatory bowel disease [Citation6–9].
The role of obesity as a risk factor for sarcoidosis has been investigated in few studies. We aimed to investigate the relationship between sarcoidosis and MetS and insulin resistance (IR). To our knowledge, this study is the first study to examine the relation of MetS and sarcoidosis.
Materials and methods
Sample of the study population
In our study, 47 patients with sarcoidosis who applied to our outpatient clinic and 45 healthy individuals without chronic disease were included. This study was approved by the Ethics Committee with a waiver of the need to obtain informed consent. All participants’ data were obtained from our database.
MetS and IR calculation
All patients were evaluated for MetS according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATP III) criteria [Citation10] (). The presence of three of the five factors defined by ATP III for MetS was accepted as a diagnosis of MetS.
Table 1. ATP III metabolic syndrome diagnostic criteria.
The body mass index (BMI) was calculated according to the weight (kg)/height (m) = kg/m2.
IR was calculated using the (HOMA-IR) index [Citation11]. It was determined using serum glucose and insulin values. Blood values were evaluated after a 12-h fasting period. IR was not calculated in patients with DM and under DM treatment. IR values greater than 2.5 were considered positive: IR: fasting insulin × fasting glucose/405.
Statistical analysis
SPSS-21 program was used for statistical analysis. Descriptive statistics were used. Odds ratio values were calculated by performing Chi-square and risk analysis for MetS and IR in sarcoidosis patients. p < .05 was considered statistically significant.
Results
The mean age of the 47 patients with sarcoidosis was 50.7 ± 12.2 years and the mean age of the 45 control groups was 42.9 ± 14.4 years. Thirty-six sarcoidosis patients were female (76.6%), 11 (23.4%) were male. Gender distribution of controls was compatible to the study group (female/male: 27/18, 60%/40%, p = .068). General characteristics and biochemistry values related to MetS are shown in .
Table 2. General characteristics of sarcoidosis and control group, biochemistry values related to MetS.
Mean age, BMI, waist circumference, triglyceride, and glucose values of patients with sarcoidosis were significantly higher than those of the controls.
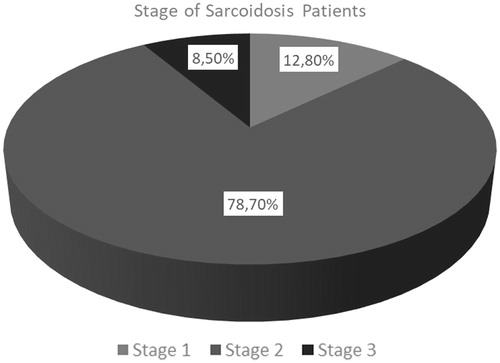
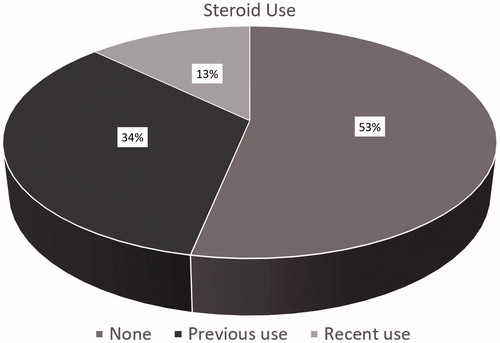
Distribution of the patients according to the stages of sarcoidosis is shown in . Almost 80% of the patients were diagnosed as stage 2 sarcoidosis. Distribution of the patients according to the use of steroid is shown in . Almost half of the patients received steroid previously or recently.
Patients with sarcoidosis have a 7.66 relative risk for MetS, whereas they also have a 5.48 relative risk of IR development ().
Table 3. Relative risk ratios for metabolic syndrome and insulin resistance among sarcoidosis patients.
Discussion
Sarcoidosis is a multisystem granulomatous disorder that most often affects the lungs and may cause significant morbidity. Sarcoidosis can manifest as neurological disease, uveitis, blindness, end-stage pulmonary fibrosis, pulmonary hypertension, dysrhythmias, cardiomyopathy, hypercalcemia, and renal failure. While the etiology of sarcoidosis is still unknown, recent insights into its immunopathogenesis have moved investigators closer to find more effective treatments [Citation12].
This study showed that the frequency of MetS and IR was increased in patients with sarcoidosis compared to the control group. Obesity; both as a result of sarcoidosis treatment and as a result of the pro-inflammatory environment of obesity. Obesity can occur as a result of sarcoidosis treatment, similarly the pro-inflammatory environment of obesity may contribute to the development of sarcoidosis.
Immunological mechanisms involved in the pathogenesis of sarcoidosis and MetS considered these two diseases may be the cause or result by each other [Citation6, Citation13]. In our study, we found that the etiology of sarcoidosis is unknown. It is thought that there is an immunological over-response against unknown antigens in people with genetic background [Citation14]. Antigens which are phagocytosed by macrophages and dendritic cells known as antigen-presenting cells (ASHs) creates epitopes on the surface of the ASH that can match with the MHC (major histocompatibility complex) class II found on the surface of T lymphocytes. As a result of this pairing, natural T lymphocytes are transformed into the Th1 phenotype and many cytokines and chemokines are secreted, leading to the development of sarcoid granuloma formation [Citation15].
Chronic low-grade inflammation invariably accompanies MetS and is associated with adverse proinflammatory cytokine profile both in serum and adipose tissue [Citation16]. Obesity is an important criterion of MetS. Laboratory studies support the possibility that obesity and weight gain increase inflammation. Pohl et al. observed enhanced and prolonged inflammatory responses to acute systemic challenge with lipopolysaccharide in diet-induced obese adult rats [Citation17]. Similarly, challenged rats that experienced induced cumulative weight gain from birth to adulthood resulting in adult overweight or obesity were found to have elevated plasma levels of proinflammatory cytokines, including tumor necrosis factor-a, IL-1 beta, and IL-6 [Citation18].
Today, obesity is considered as a low-grade systemic inflammatory condition including high levels of inflammatory markers such as leptin, C-reactive protein, tumor necrosis factor-a, and interleukin-6 [Citation19]. The etiology of sarcoidosis is unknown, but the disease involves immunological changes similar to those seen in obesity, including the production of TNF-α. Obesity-induced changing immunology may have a role in the development of sarcoidosis [Citation9].
Sarcoidosis is an autoimmune disease [Citation20]. There are studies suggesting that autoimmune diseases (rheumatoid arthritis, psoriasis, autoimmune thyroid diseases) increase with BMI increase [Citation21–26]. Hapsoe et al. in a study investigating BMI and autoimmune diseases; 75,008 women have been followed for 11 years. The relationship between BMI and 43 autoimmune diseases was investigated; 2430 women developed autoimmune disease and 140 of them developed sarcoidosis. It was stated that obesity increased the risk of sarcoidosis three times [Citation20].
In Dumas et al., 116,430 women were followed up for 14 years and 270 cases developed sarcoidosis. There was a positive correlation between BMI and sarcoidosis patients [Citation27]. Similarly, in our study, the BMI of sarcoidosis patients was significantly higher than the control group.
A study made around African-American women has found that obesity is associated with a higher risk of sarcoidosis in the long term. A subsequent study involving data from The Black Women’s Health Study, a follow-up study of 59,000 African-American women, also prospectively assessed the association between obesity and incident sarcoidosis over a 16-year follow-up period (1995–2011) during which 454 cases were reported. Obesity at study baseline (1995) was associated with a 42% increased incidence of sarcoidosis [Citation13].
It is well known that white adipose tissue are not only a storage organ, but play an active role in producing and releasing various mediators used in physiological processes [Citation28,Citation29]. These mechanisms may simultaneously trigger autoimmunity that leads to sarcoidosis.
Of all the ADs, the highest risk (more than threefold) in obese women was observed for sarcoidosis, a disease that, to our knowledge, has not previously been related to obesity besides increased BMI in patients with existing sarcoidosis. In a clinical study of 184 patients with sarcoidosis and age- and sex-matched control subjects, higher BMI was associated with lower FVC %, higher fatigue, and poorer self-reported health status [Citation30].
Leptin is an appetite-regulating peptide hormone that is synthesized from white adipose tissue. The leptin value in the blood is directly proportional to adipose tissue [Citation31,Citation32]. It has been reported that leptin receptors are expressed on B and T cells and leptin has direct effects on lymphocytes. Leptin can stimulate T cell proliferation, stimulate a Th1 response, affect T cell activation, and increase phagocytic activity by activating macrophages and monocytes. In animal models, leptin deficiency has been associated with immune suppression [Citation33]. Sarcoidosis is characterized by chronic granulomatous inflammation, which is believed to be a result of a persistent T-helper 1 polarized immune response [Citation1]. Obesity and leptin may trigger the development of sarcoidosis by creating T Helper response.
Systemic steroid therapy may cause weight gain in patients [Citation34]. Steroid treatment may be used in some clinical conditions in patients with sarcoidosis. More than half of our patients were not on steroid treatment.
It was considered in obesity that macrophage increase in adipose tissue results in metabolic inflammation. Chronic, subacute inflammatory conditions accompanied by obesity affects the adipose tissue, liver, and vascular system. Obesity-induced inflammatory changes occur in both immune and non-immune cells. Then inflammation leads to the abnormal production of cytokines and chemokines by activating immune cells [Citation35]. Research in obese rodents has shown that increase in adipocyte-induced TNF-a will result in systemic IR [Citation35,Citation36].
Nishimura et al. showed that obesity increase the number of CD8 + [Citation35]. MetS is well known to be associated with a high inflammatory condition [Citation37,Citation38]. Inflammatory molecules such as C-reactive protein (CRP), TNF-alpha, plasma resistin, IL-6, and IL-18 are found to be significantly increased in adipose tissue of MetS [Citation37]. The degree of expression of TNF-alpha in adipose tissue was found to be directly proportional to plasma insulin value [Citation37,Citation39]. Plasma TNF is also associated with fasting insulin and IR (HOMA) [Citation37,Citation39,Citation40]. Therefore, it can be said that, TNF-alpha plays a role in the pathogenesis of IR.
IL-10 is a major anti-inflammatory cytokine associated with IR, obesity, MetS, and Type 2 DM [Citation41]. Serum IL-10 levels were significantly correlated with IL-6, CRP, and TNF-alpha levels, but did not correlate with adiponectin in healthy subjects. Therefore, IL-10 is significantly associated with adiponectin, especially in subjects with MetS [Citation42]. For this reason, IL-10 may be play a role in the inflammatory process of MetS [Citation37,Citation42].
Pleiotropic proinflammatory cytokine IL-18 plays a role in the inflammatory cascade that promotes the production of both TNF and IL-6 [Citation37]. It has been reported that there is an inverse relationship between IL-18 and antiatherogenic adipokine adiponectin in obesity, IR, CVD, and MetS [Citation37,Citation43].
In conclusion, this study show that MetS is associated with increased sarcoidosis risk. MetS and IR diagnosis was higher in patients with sarcoidosis. The question is unclear: Is MetS risk factor for sarcoidosis? or Is sarcoidosis risk factor for MetS? MetS, which is an important cause of morbidity and mortality, has a high rate of sarcoidosis, suggests that it may play a role in the pathogenesis of that disease.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165.
- Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735–737.
- Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–1234.
- Alberti KG, Eckel RH, Grundy SM Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes federation task force on epidemiology and prevention. Circulation. 2009;120:1640–1645.
- Jensen MD, Ryan DH, Apovian CM. 2013AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:102–138.
- Cozier YC, Govender P, Berman JS. Obesity and sarcoidosis: consequence or contributor? Curr Opin Pulm Med. 2018;24:487–494.
- Crowson CS, Matteson EL, Davis JM, 3rd, et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65:71–77.
- Love TJ, Zhu Y, Zhang Y, et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis. 2012;71:1273–1277.
- Harpsoe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43:843–855.
- Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48.
- Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care. 2009;32:2297–2299.
- Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011;305:391.
- Cozier YC, Coogan PF, Govender P, et al. Obesity and weight gain in relation to incidence of sarcoidosis in US black women: data from the Black Women's Health Study . Chest. 2015;147:1086–1093.
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2013;385:1155–1167.
- Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med. 2008;29:379–390.
- Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and T2D. Diabetes Res Clin Pract. 2014;105:141–150.
- Pohl J, Woodside B, Luheshi GN. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rat. Endocrinology. 2009;150:4901–4910.
- Clarke MA, Stefanidis A, Spencer SJ. Postnatal overfeeding leads to obesity and exacerbated febrile responses to lipopolysaccharide throughout life. J Neuroendocrinol. 2012;24:511–524.
- Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953.
- Dagur PK1, Biancotto A, Wei L, et al. MCAM-expressing CD4(+) T cells in peripheral blood secrete IL-17A and are significantly elevated in inflammatory autoimmune diseases. J Autoimmun. 2011;37:319–327.
- Matarese G, Leiter EH, La C. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70:87.
- Symmons DP, Bankhead CR, Harrison BJ, et al. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis: results from a primary care-based incident case-control study in Norfolk, England. Arthritis Rheum. 1997;40:1955.
- Voigt LF, Koepsell TD, Nelson JL, et al. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5:525–532.
- Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, et al. Obesity in rheumatoid arthritis. Rheumatology (Oxford). 2011;50:450.
- (a) Duntas LH, Biondi B. The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid 2013;23:646–653. (b) Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes 2012;2:e54.
- Sterry W, Strober BE, Menter A. Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol. 2007;157:649.
- Dumas O, Boggs KM, Cozier YC, et al. Prospective study of body mass index and risk of sarcoidosis in US women. Eur Respir J. 2017;50:1701397.
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–920.
- Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14:1095–1100.
- Gvozdenovic BS, Mihailovic-Vucinic V, Vukovic M, et al. Effect of obesity on patient-reported outcomes in sarcoidosis. Int J Tuberc Lung Dis. 2013;17:559–564.
- Dornbush S, Aeddula NR. Physiology, Leptin. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019.
- Gruzdeva O, Borodkina D, Uchasova Dyleva EY, et al. Leptin resistance: underlying mechanisms and diagnosis. Diabetes Metab Syndr Obes. 2019;12:191–198.
- Otero M, Lago R, Gomez R, et al. Towards a pro-inflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford). 2006;45:944–950.
- Dağ ZÖ, Dilbaz B. Impact of obesity on infertility in women. J Turkish German Gynecol Assoc. 2015;16:111–117.
- Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation-mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771–1776.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
- Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822.
- Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord. 2004;2:82–104.
- Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alfa in human obesity and insülin resistance. J Clin Invest. 1995;95:2409–2415.
- Xydakis AM, Case CC, Jones PH, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. 2004;89:2697–2703.
- Ye JH, Li ZZ, Li Y, et al. [Relationship between serum interleukin-10 and insulin resistance in metabolic syndrome]. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:428–430.
- Nishida M, Moriyama T, Sugita Y, et al. Interleukin-10 associates with adiponectin predominantly in subjects with metabolic syndrome. Circ J. 2007;71:1234–1238.
- Straczkowski M, Kowalska I, Nikolajuk A, et al. Increased serum interleukin-18 concentration is associated with hypoadiponectinemia in obesity, independently of insulin resistance. Int J Obes. 2007;31:221–225.