Abstract
Background
Skeletal muscle is an important site for storing proteins and providing general physical function. Recent research has shown that muscle strength decreases earlier than muscle mass decreases, as shown during the aging process. Our article aimed to compare the association between testosterone levels and grip strength to provide an earlier biomarker to detect muscle weakness.
Method
We adopted quartile-based analysis by dividing handgrip power into quartiles, with all participants in the lowest quartile serving as the reference group. Linear regression analysis was conducted between handgrip power and testosterone. Logistic regression models were used to analyze the longitudinal correlation between testosterone levels and the presence of low muscle strength.
Results
Serum testosterone levels had a significant correlation with grip strength in all models (p < .001). In addition, high testosterone levels were negatively associated with low muscle strength in all groups (p < .001). A stronger relationship was observed between testosterone levels and grip strength among non-obese participants than among obese participants.
Conclusions
In conclusion, our study highlighted that testosterone levels are related to greater grip strength.
Keywords:
Introduction
The aging process involves several critical changes. The most evident change includes a loss of strength and the ability to care for oneself, causing disability, frailty, and a decreased quality of life [Citation1]. Consequently, much research has focused on identifying strategies to maintain muscle mass and muscle strength during the aging process. However, it is not sufficient to define sarcopenia by the amount of muscle mass because there is no consensus on the degree of change in muscle mass and the method of measuring muscle mass. Most importantly, recent research has demonstrated that muscle strength is not associated with muscle mass due to physiological factors [Citation2]. Many articles have indicated that the decrease in grip strength was greater than the predicted decrease from the previous cross-sectional study. Therefore, with several important consensus meetings, the operational definition of sarcopenia gradually progressed to include both muscle quality and muscle mass, and it is also expected to have clinically practical cut points. For the assessment of sarcopenia, it is currently recommended to measure muscle mass using dual-energy X-ray absorptiometry (DXA). On the other hand, we used age-adjusted grip power to represent muscle strength. The isometric strength of the handgrip test could be quantified by squeezing on a dynamometer. This examination assessed the maximal power of the hand and forearm muscles. Grip strength is indispensable in our daily activities, such as grasping, twisting, picking up, or lifting objects. Grip strength has been used as a marker of nutritional status, activities of daily living, and subsequent disability [Citation3–5].
Testosterone deficiency during the aging process is characterized by distinct features and a low level of serum testosterone. Lower sexual desire and low urinary tract symptom are correlated with adult testosterone levels [Citation6]. Especially, a Longitudinal Study presented that dropped testosterone level had the significant relationship with prostate cancer [Citation7]. Recently, many surveys have indicated that declining muscle mass, muscle strength, disability, and reduced physical performance have a significant positive relationship with testosterone deficiency, and the correlation between grip strength and decreasing serum total testosterone has been recognized using only freely available data [Citation8].
We hypothesized that serum testosterone concentration was related to grip strength in adults. We designed a study using original data from the National Health and Nutrition Examination Survey (NHANES) to delineate the association between grip strength and testosterone.
Materials and methods
Participants
For health policies and national health surveys, the NHANES survey has been performed on more than 100,000 Americans since 1960. It has become a continuous form of data collection since 1999. Most of the study included a detailed interview performed at a mobile examination center (MEC). The contents of the NHANES questionnaires, physical examination documents, and laboratory specimen sampling are available for download and analysis for free on the NHANES webpage. We adopted the datasets from NHANES from 2011 to 2012, which included a representative sample of demographic, examination, laboratory, and questionnaire datasets with multistage probability sampling. All individuals over 20 years of age with data on grip strength and testosterone levels were included in our analysis. The collection of NHANES data is validated by the National Center for Health Statistics Research Ethics Review Board. Informed consent was obtained from all individuals before the exam began. The data from 7064 screened participants in NHANES 2011–2012 were eligible for analysis.
Measurement of combined grip strength
The combined grip strength test protocol consisted of the NHANES Muscle Strength and Procedural Manual. Handgrip dynamometers were calibrated periodically before every test, and all examiners were well-trained in the examination procedures. The results were also reliable between examiners and within multiple attempts by the same examiner. The subject tested the dynamometer by holding it with the arm and elbow perpendicular to the ground. Every participant squeezed the device with the maximal isometric force and maintained this force for 5 s. The bilateral grip strength of each included participant was measured every 60 s, and we summed the measurements from the three trials to represent the overall result for the standard grip strength dynamometer. We measured grip strength to determine muscle strength. Patients in the lowest quartiles for grip strength in our study were defined as having low muscle strength.
Measurement of total testosterone
We used isotope–dilution liquid chromatography–tandem mass spectrometry (ID–LC–MS/MS) for testosterone to serve higher sample throughput with excellent accuracy [Citation9]. The reliability rate over 2 years was 95.2%.
Covariates
Age, race, and smoking status were collected by self-report questionnaires. Anthropometric variables, such as weight, height, and waist circumference, were gathered during the physical checkup according to the NHANES protocol. Body mass index (BMI) was calculated by dividing individuals’ weight by the square of their height (kg/m2). Albumin, alanine transaminase, and serum glucose concentrations were measured by an electronic chemistry analyzer. Information on the comprehensive collection, storage, and processing of biospecimens is accessible on the NHANES website. Average systolic blood pressure was calculated with three blood pressure measurements taken with a mercury sphygmomanometer by an NHANES examiner. Finally, self-reported cardiovascular problems, such as coronary artery disease (CAD), congestive heart failure (CHF), Angina_pectoris (AP), and heart attack, were comorbidities included in the adjustment for baseline covariates.
Statistics analysis
Some variables were considered to be continuous variables, such as age, combined grip strength, BMI, waist circumference, systolic blood pressure, testosterone, albumin, alanine aminotransferase, creatine phosphokinase, and serum glucose. On the other hand, some of the covariates were regarded as categorical variables, including race, smoking, and comorbidities. We adopted quartile-based analysis by dividing handgrip power into quartiles, with all participants in the lowest quartile serving as the reference group. The cutoff values for handgrip power quartiles were as follows: Q1 < 44.7 kg, 44.7 kg ≤ Q2 < 59.6 kg, 59.6 kg ≤ Q3 < 80.3 kg, and Q4 ≥ 80.3 kg. Linear regression analysis was conducted between handgrip power and testosterone. An extended-model method included the following variable adjustments: Model 1 = age, BMI, and race/ethnicity; Model 2 included the unadjusted subjects (Model 1) combined with other covariates, such as waist circumference, systolic blood pressure, albumin, alanine aminotransferase, creatine phosphokinase, and serum glucose; and Model 3 = Model 2 with the inclusion of the following items: habit of smoking and histories cardiovascular diseases, such as CHF, CAD, AP, and heart attack. All analyses were conducted using SPSS, 18th edition.
Results
Characteristics of the study population
A total of 7064 participants is described in . Compared with the participants who had weak grip strength, those with strong grip strength were more likely to be older, male, non-Hispanic white or black American, and physically active. In addition, participants tended to have a larger waist circumference, higher SBP, and high levels of serum albumin, alanine aminotransferase, creatinine phosphokinase, and glucose (p < .001).
Table 1. Characteristics of study participants.
Association between total testosterone levels and levels of grip strength
First, grip strength was correlated positively with testosterone (beta coefficient: 6.244; 95% CI: 5.982, 6.506; p < .001) in the unadjusted model (). Similar results were observed after multivariate adjustment in all the models. Furthermore, and demonstrate the findings of quartile-based multiple linear regression analysis of handgrip power to determine the direct associations between grip strength and testosterone. Those with greater grip strength tended to have higher levels of testosterone, thus showing a prominent association between grip strength and testosterone levels.
Figure 1. Relationship between the quartiles of grip strength and serum testosterone level in all participants.
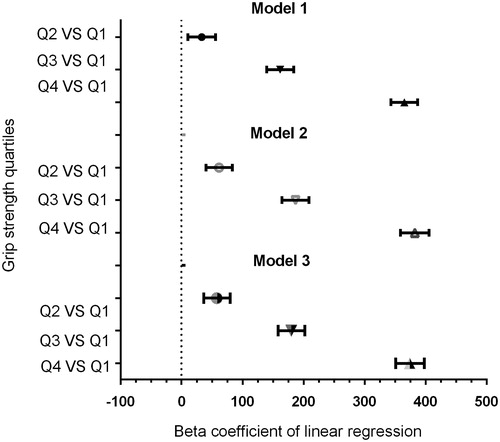
Table 2. Association between testosterone level and grip strength.
Relationships between testosterone and grip strength categorized by obesity
In , we further examined the association between testosterone levels and grip strength according to BMI. The data showed that testosterone levels were related to grip strength in both the obese and non-obese groups. However, a stronger association was observed between testosterone concentration and grip strength among non-obese participants than among obese participants.
Table 3. The association between testosterone level and grip strength in non-obese (BMI < 30) and obese (BMI ≥ 30) men.
In the quartiles-based regression analysis (), positive associations between testosterone levels and grip strength were noted in all models; the same associations were found in the obese group. However, no significant differences between testosterone levels and grip strength were found in Q2 vs. Q1 in model 1 in the obese group (beta coefficient: 21.455; 95% CI: −7.530, 50.440; p = .147).
Table 4. The association between testosterone level and grip strength in non-obese (BMI < 30) and obese (BMI ≥ 30) men.
Association between total testosterone level and low muscle strength
In the multivariate logistic regression (), individuals with higher testosterone levels had a lower risk of low muscle strength (OR: 0.981; 95% CI: 0.979, 0.983; p < .001). Participants were categorized by BMI in each model. In unadjusted models, those with a BMI < 30 showed that testosterone levels were negatively associated with low muscle strength (OR: 0.981; 95% CI: 0.979, 0.983; p < .001). Taken together, testosterone levels were negatively related to low muscle strength in obese or non-obese individuals.
Table 5. Association between testosterone level and low muscle strength.
Discussion
The most important result in this study was a positive association between serum testosterone levels and grip strength in the higher quartiles in all models, even after adjustments of pertinent variables. When we divided participants into quartiles based on grip strength to determine the association between grip strength and testosterone levels, a dose-dependent relationship was evident. Notably, testosterone and low muscle strength were negatively correlated. Notably, the results showed a lower correlation between testosterone levels and grip strength among obese individuals than among non-obese individuals.
A growing number of studies have investigated the association between serum testosterone levels and grip strength [Citation10,Citation11]. In a cross-sectional study of 144 patients who had undergone kidney transplantation in Germany, testosterone concentration was associated with grip strength and C-reactive protein and albumin levels. Another cross-sectional study of 1489 older men by TungWai Auyeung found that high serum testosterone concentration was correlated with not only greater muscle power and mass but also better physical functioning. Similarly, a study [Citation12] showed that lower levels of sex hormones in men were accompanied by impaired physical functioning and weak muscle power. Conversely to our study with healthy participants, Loncar et al.’s study [Citation13] declared that the testosterone level had no correlation with grip strength in patients with heart failure. Another study by Wu et al. found that elderly individuals with low testosterone levels were positively associated with frailty [Citation14]. In contrast to previous research, our study was the first to demonstrate a positive correlation between serum testosterone levels and grip strength, not only in the healthy group but also in several samples (7064 people). Most importantly, our participants included relatively healthy and young people (the average age was 36.21 years old). We can infer that even in relatively young and healthy populations, a decline in testosterone levels still caused a loss of muscle strength, which affected physiological functioning and quality of life. People usually thought that the aging process led to a distinct loss of muscle strength. However, the evidence suggested otherwise. In other words, the process of aging occurs earlier than we expected.
In , we focused on the relationship between testosterone levels and low muscle strength. The related previous article showed that serum testosterone levels were similar to those of individuals with increased skeletal muscle strength and protein synthesis [Citation15]. The result was in line with the findings of our analysis indicating that the higher the levels of testosterone are, the lower the risk of muscle weakness.
In particular, there had been few articles discussing the relationship between dynapenia, obesity, and testosterone levels. A 3-year cross-sectional study [Citation16] involving individuals 55 years of age and older from NHANES revealed that individuals with dynapenic obesity had poorer physical activity than those with dynapenia or obesity alone. In a cross-sectional study, Blaya et al. demonstrated that abdominal obesity was correlated to low testosterone level [Citation17].
Bouchard and Janssen [Citation18] were convinced that both obesity and low muscle strength played an important role in physical performance. Moreover, they believed that these two factors had an additive instead of a multiplicative effect. This conclusion was also consistent with the findings of our study that the correlation between testosterone concentration and grip strength of obese individuals is lower than that of non-obese individuals.
Testosterone was a type of physiological anabolic hormone. The decrease in serum testosterone levels confirmed an extrinsic factor as one of the common reasons for sarcopenia [Citation19]. Practically, testosterone replacement therapy could be considered to enhance the anabolic effects that stimulate the growth of muscle strength and mass [Citation20]. Emerging evidence addressed that testosterone supplement therapy was still controversial [Citation21]. The related study published by Lawrence showed that short-term testosterone supplementation enhanced resistance exercise in older men [Citation22]. Krause Neto et al. presented that the mice in resistance training with anabolic steroid (RTA) group had the greater maximum carried load than resistance training (RT) group [Citation23]. Testosterone supplementation generated greater nutrition and oxygenation of the organ due to increasing collagen and microvascularization [Citation24]. Studies thus far had mentioned that plasma testosterone concentration accelerates assimilation by short- and long-term mechanisms to expedite protein synthesis by increasing ribosome quantity [Citation25]. In large animal experiments, one researcher found that castrated mice had smaller-sized motor neurons than did common mice. The author inferred that hypogonadism had adversely affected developing motor neurons. Therefore, they had poor quality and efficiency of muscle function compared to common mice [Citation26,Citation27]. On the other hand, low serum androgen concentrations had also been demonstrated to be related to an increase in systematic inflammation [Citation28]. Many had identified testosterone supplement therapy in reducing C-reactive protein levels. That is, testosterone had a negative association with chronic inflammation [Citation29,Citation30]. In a 5-year longitudinal study, Schaap proved that chronic low-grade inflammation, such as IL-6 and TNF-a, would decrease muscle mass and grip strength [Citation31]. On the other hand, testosterone altered skeletal muscle mass and strength by intrinsic mechanisms such as mitochondrial biogenesis. The research published by Guo showed that the serum testosterone levels not only upgraded the quality of mitochondrial proteins but also lessened the oxidative stress of tissues. As mentioned above, testosterone could lead to the activation of pro-myogenic and anti-inflammatory growth factors [Citation32]. Initially, cross-sectional studies [Citation33] evaluating the correlation between muscle power and muscle mass demonstrated a difference of approximately 35% difference in healthy people. In recent research, however, the reduction in muscle power was clearly faster than the accompanying muscle depletion. In addition, muscle strength continued to fall even when muscle mass was preserved or increased [Citation34]. In his experiment, Clark found that muscle disuse had little impact on strength. In contrast, low muscle strength played a critical role in motor malfunction and poor physical functioning [Citation35]. The most recent article demonstrated that grip strength divided by body weight was a measure that was markedly relevant to physical functioning. Moreover, among non-dynapenic and dynapenic individuals, the former had positively and prominently superior physical functioning in a group of postmenopausal women [Citation36]. A similar conclusion of a high correlation between grip strength and dynapenia appeared in different samples in other studies [Citation36–38]. Grip strength was an effective overall measure of serum testosterone concentration and had been used to evaluate sexual behavior, body morphology, and aggression in specific groups in recent research [Citation39,Citation40]. The Supplement Table 1 lists the latest articles examining serum testosterone levels and grip strength.
The conclusion regarding the relationship between testosterone levels and grip strength was clinically useful; however, there were some limitations to our study. First, this was a retrospective study of a cross-sectional database without an intervention, which limited causality and causal inferences. We did not evaluate the relationship between grip strength and testosterone levels over time because we only measured handgrip force once instead of tracking it over time. It was unreasonable to seek causality between hand strength and testosterone levels in a cohort study. Second, our investigation was controlled for some potential confounding factors. Other variables, such as inflammatory status, daily physical performance, neurological function, or other comorbidities, were not completely adjusted for analyses. Third, our study used data from NHANES, which might have led to a sampling bias and limited our findings to healthy Americans who can walk. Finally, we accessed the information for the subjects’ medical history by questionnaire; thus, recall bias should be considered.
Conclusions and implications
Our study displayed evidence that greater grip strength was strongly associated with higher testosterone levels in younger and healthier individuals. Similarly, people with high testosterone had a lower risk of low muscle strength. A previous study proved that age-related reductions in testosterone levels were related to low muscle power in older subjects. However, our literature review showed that the same condition was found in young or middle-aged individuals. Undoubtedly, the process of aging begins earlier than we expected. Evaluating grip strength throughout early life might be useful in identifying early hypogonadism among individuals from a health promotion perspective. We expected the development and clinical application of simple and executable criteria for grip strength in the future to aid in the initial predictions of early deficiencies in serum testosterone levels. Through testosterone supplement therapy was still controversial, some articles had noted its benefits to some populations. It will serve as a novel indicator to screen for latent testosterone deficiency.
Supplemental_Table_1.docx
Download MS Word (16.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- Perez-Zepeda M, Cesari M, Carrillo Vega MF. A frailty index from next-of-kin data: a cross-sectional analysis from the Mexican health and aging study. BioMed Res Int. 2017;2017.
- Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834.
- Norman K, Stobaus N, Gonzalez MC, et al. Hand grip strength: outcome predictor and marker of nutritional status. Clini Nutr. 2011;30:135–142.
- Giampaoli S, Ferrucci L, Cecchi F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288.
- Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199:257–265.
- Xu X, Zhang X, Zhong Y, et al. Dynamic patterns of testosterone levels in individuals and risk of prostate cancer among hypogonadal men: a longitudinal study. J Urol. 2018;199:465–473.
- Page ST, Amory JK, Bowman FD, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metabol. 2005;90:1502–1510.
- Tai SS, Xu B, Welch MJ, et al. Development and evaluation of a candidate reference measurement procedure for the determination of testosterone in human serum using isotope dilution liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem. 2007;388:1087–1094.
- Auyeung TW, Lee JS, Kwok T, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross-sectional study in 1489 older men. Eur J Endocrinol. 2011;164:811–817.
- Gurlek Demirci B, Sezer S, Tutal E, et al. Hand-grip strength is associated with serum testosterone and albumin levels in male kidney transplant recipients. Exp Clin Transplant. 2018;16(Suppl 1):75–79.
- Schaap LA, Pluijm SM, Smit JH, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf). 2005;63:152–160.
- Loncar G, Bozic B, Neskovic AN, et al. Androgen status in non-diabetic elderly men with heart failure. Aging Male. 2017;20:215–224.
- Wu IC, Lin XZ, Liu PF, et al. Low serum testosterone and frailty in older men and women. Maturitas. 2010;67:348–352.
- Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826.
- Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:71–77.
- Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male. 2016;19:85–89.
- Bouchard D, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2009;65:71–77.
- Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. World J Mens Health. 2018;36:192–198.
- Sinha-Hikim I, Artaza J, Woodhouse L, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–64.
- Zhang X, Zhong Y, Saad F, et al. Clinically occult prostate cancer cases may distort the effect of testosterone replacement therapy on risk of PCa. World J Urol. 2019.
- Judge LW, Bellar DM, Hoover DL, et al. Effects of acute androstenedione supplementation on testosterone levels in older men. Aging Male. 2016;19:161–167.
- Krause Neto W, de Assis Silva W, Polican Ciena A, et al. Total training load may explain similar strength gains and muscle hypertrophy seen in aged rats submitted to resistance training and anabolic steroids. Aging Male. 2018;21:65–76.
- Goncalves L, de Souza RR, Maifrino LB, et al. Resistance exercise and testosterone treatment alters the proportion of numerical density of capillaries of the left ventricle of aging Wistar rats. Aging Male. 2014;17:243–247.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63:280–293.
- Fraley GS, Ulibarri CM. Long-term castration effects motoneuron size but not number in the spinal nucleus of the bulbocavernosus in the adult male Mongolian gerbil. Brain Res. 2002;953:265–271.
- Park JJ, Zup SL, Verhovshek T, et al. Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and BCL-2 overexpressing mice. J Neurobiol. 2002;53:403–412.
- Mohamad N-V, Ima-Nirwana S, Chin K-Y, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318.
- Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602.
- Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189.
- Guo W, Wong S, Li M, et al. Testosterone plus low-intensity physical training in late life improves functional performance, skeletal muscle mitochondrial biogenesis, and mitochondrial quality control in male mice. PLoS One. 2012;7:e51180.
- Frontera WR, Hughes VA, Lutz KJ, et al. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71:644–650.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A, Biol Sci Med Sci. 2006;61:1059–1064.
- Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28:495–503.
- Dulac M, Boutros GE, Pion C, et al. Is handgrip strength normalized to body weight a useful tool to identify dynapenia and functional incapacity in post-menopausal women? Braz J Phys Ther. 2016;20:510–516.
- Bohannon RW, Magasi S. Identification of dynapenia in older adults through the use of grip strength t-scores. Muscle Nerve. 2015;51:102–105.
- Iwamura M, Kanauchi M. A cross-sectional study of the association between dynapenia and higher-level functional capacity in daily living in community-dwelling older adults in Japan. BMC Geriatr. 2017;17:1.
- Gallup AC, O'Brien DT, White DD, et al. Handgrip strength and socially dominant behavior in male adolescents. Evol Psychol. 2010;8:229–243.
- Gallup AC, White DD, Gallup GG. Jr. Handgrip strength predicts sexual behavior, body morphology, and aggression in male college students. Evol Hum Behav. 2007;28:423–429.
