Abstract
Objective
The aim of this study was to investigate whether concomitant treatment of dutasteride and sildenafil could prevent structural changes in the penis of a BPH rodent model.
Methods
Thirty-two adult male rats were divided into the following groups: Ctrl, untreated control rats; BPH, untreated spontaneously hypertensive rats (SHRs); BPH + D, SHRs treated with dutasteride; and BPH + DS, SHRs treated with dutasteride and sildenafil. All treatments were performed during 40 days, following which the penises were collected for histomorphometrical analysis. The results were compared via one-way ANOVA with Bonferroni’s post-test, considering p values <.05 as significant.
Results
The smooth muscle density decreased by 28.6% and 21.4% in BPH + D and BPH + DS, respectively, when compared to the BPH group. The sinusoid space density reduced by 32.2% in BPH, when compared to the Ctrl group; this density was also reduced by 22.6% in BPH + D, when compared to the BPH group. The density of the elastic fibers increased 51.6% and 65.6% in BPH + D and BPH + DS, when compared to the BPH group.
Conclusion
Treatment with dutasteride promoted morphological changes in the corpus cavernous of this BPH model. Concomitant treatment with sildenafil did not prevent the morphological changes caused by dutasteride; on the contrary, it also promoted a further increase in elastic fibers.
Introduction
Benign prostatic hyperplasia (BPH) is a highly prevalent condition that may affects 50% of men after 50 years and 90% of men after the eighth decade of life [Citation1,Citation2]. Lower urinary tract symptoms (LUTS) associated with BPH is associated with a decrease on patients’ quality of life, as well as some of the routinely used treatments [Citation3].
Dutasteride is a 5-α-reductase inhibitor (5ARI) used as first line treatment for BPH [Citation4,Citation5]. This drug inhibits type I and II isoforms of the enzyme, which is responsible for the conversion of testosterone (TT) in dihydrotestosterone (DHT) [Citation6]. As DHT is the most active androgen hormone, the reduced levels of this hormone is associated with prostatic volume reduction [Citation7–10]. However, DHT is also responsible for other metabolic functions, and treatment with 5ARI is associated with adverse effects such as erectile dysfunction (ED) decreased libido and decreased ejaculation [Citation11–13].
Some studies propose concomitant treatment with 5ARI and phosphodiesterase-5 inhibitors (PDE5I) to prevent ED caused by the low DHT levels [Citation14–16]. Sildenafil is the most used drug for ED treatment [Citation17,Citation18]. It increases the synthesis of nitric oxide, relaxing the endothelial smooth muscle of the corpus cavernosum (CC), and increasing blood flow in the sinusoidal spaces [Citation19,Citation20].
Our group recently showed that the penis of a rodent BPH model treated with dutasteride presents several morphological modifications [Citation21]. In another study, it was shown that sildenafil treatment preserved the normal CC structure in hypertensive animals (which was altered in untreated animals) [Citation22]. Thus, the hypothesis of this study is that the treatment with a PDE5I may prevent penile morphological alterations associated with 5ARI. The aim of this study was to investigate the penile morphology in a BPH rodent model treated with dutasteride alone or associated with sildenafil.
Material and methods
Animal model and experimental design
The spontaneously hypertensive rat (SHR) strain was developed by inbreeding from Wistar Kyoto rats. These animals are largely used for studying arterial hypertension, and its effects on different organs and treatments. More recently, the SHR strain has been proposed as a model for studying BPH as these animals showed prostatic morphological alterations [Citation23], as well as LUTS. Thus, the SHR strain was used as a model of BPH in this study, and the Wistar Kyoto strain was used as control animals.
Twenty-four male SHRs and eight male Wistar Kyoto rats were used in this study. All animals were created in our laboratory and were included in the experiment with four months of age. They were kept in a room with a controlled temperature, artificial dark–light cycles, and had free access to standard rat food and water. This project followed international guidelines for animal experimentation and was formally approved by the local ethics committee under the protocol number CEUA-051/2012.
All animals underwent a washout period from 3 to 4 months of age, when water was administered daily by gavage and blood pressure was assessed weekly. Further, during the experiment period, blood pressure was assessed weekly by pletismography to corroborate that SHR strain developed its expected hypertension and Wistar Kyoto strain was normotensive.
In addition, after euthanasia, the ventral prostate was collected and processed to verify the development of morphological modifications in SHRs compatible with BPH and if the treatment with dutasteride was able to ameliorate the changes in the morphology of the prostate of these animals [Citation21].
The Wistar Kyoto animals formed the control group (Ctrl, n = 8) which received distilled water. Animals of the SHR strain were randomly assigned into 3 groups: BPH group (n = 8) which received distilled water; BPH + D group (n = 8) composed of rats with BPH treated with dutasteride (Dastene 0.5 mg/Kg/day, Aché, Indaiatuba, Brazil) [Citation21,Citation24]; and BPH + DS group (n = 8) composed of rats with BPH treated with dutasteride [Citation21,Citation24]; and sildenafil (Sildenafil 30 mg/kg/day, Primordium, Rio de Janeiro, Brazil) [Citation22]. All drugs were given by gavage (diluted to the same final volume), during 40 consecutive days.
The middle shaft of the penile and prostatic fragments were fixed in 4% buffered formaldehyde solution and processed for paraffin embedding. Prostate sections and penile cross-sections (5 µm thick) were obtained and used for histomorphometric evaluations [Citation22,Citation25,Citation26].
Evaluations
Prostate sections were stained with Masson’s trichrome, and images were captured at 600× magnification and analyzed to obtain the prostatic epithelium height as described elsewhere [Citation21,Citation27,Citation28].
The areas of the penis, corpus cavernosum (including its tunica albuginea), and the corpus cavernosum without the tunica albuginea were evaluated in Picrosirius red stained sections. For this purpose, images were captured under 20× magnification by a digital camera (Axiocam 506 color, Carl Zeiss, Jena, Germany) coupled to a stereomicroscope (Discovery V(0).8, Carl Zeiss). These parameters were measured with the “Free Hand” tool of the Image J software (version 1.45s, National Institutes of Health, Bethesda, MA, USA), and expressed in square millimeters. The area of the tunica albuginea was considered the difference in the areas of the CC with and without the tunica albuginea [Citation22].
Smooth muscle fibers surface density (Sv) was assessed in anti-alpha-actin imunnolabeled sections, captured under 400× amplifications. Connective tissue and sinusoidal space Sv were studied in Masson’s trichrome stained sections, captured under 400× magnifications. For assessing the elastic fibers Sv, Weigert's resorcin fucsin staining was used, and images were captured under 600× magnification. These images were captured by a digital camera (DP70, Olympus, Tokyo, Japan) coupled to a microscope (BX51, Olympus).
The Sv of each structure was measured by the point counting method [Citation26,Citation29,Citation30]. Briefly, a 100-point grid was superimposed over the images using the Image J software, and each structure hinted by a point was counted as smooth muscle, connective tissue, sinusoidal space, or elastic fiber. The result, expressed as a percentage, was calculated after measuring five different fields from five nonserial sections, for a total of 25 images analyzed for each animal.
Picrosirius red-stained sections were observed under polarized light at 400× to differentiate collagen types III (green) and I (red/orange) [Citation31].
Statistical analysis
One-way analysis of variance (ANOVA) with Bonferroni’s post-test was used to compare mean values, considering p < .05 as significant. All analyses were performed using GraphPad Prism software (version 5.0, San Diego, CA, USA).
Results
The prostatic epithelium height in group BPH was 41.7% higher than that in group Ctrl. The use of dutasteride or dutasteride and sildenafil restored normal prostatic epithelium height. As expected, blood pressure of all SHR animals was higher than Ctrl group. These data are presented in .
Table 1. Data of prostatic epithelium height and blood pressure in the beginning and in the end of the experiment, confirming the experimental model.
There was no statistical difference among the groups regarding the total area of the penis, area of the CC with tunica albuginea, and area of the tunica albuginea. However, the area of the CC without the tunica albuginea was reduced by 5.5% in BPH, in comparison to Ctrl group. Further, there was a reduction of 11.8% and 8.8% in BPH + D and BPH + DS, when compared to the BPH group (). All histomorphometrical data are presented in .
Figure 1. Penile histological sections of control and BPH rats with dutasteride or dutasteride and sildenafil treatment. Images A, B, C, and D were stained by Picrosirius red, captured under 20× magnification, and used for assessing the areas of the penis, corpus cavernosum (including its tunica albuginea), and the corpus cavernosum without the tunica albuginea. Images E, F, G, and H were stained by Masson’s trichrome, captured under 400× magnification, and used for assessing the connective tissue and sinusoidal space surface densities. Images I, J, K, and L were immunolabeled with anti-alpha-actin antibodies, captured at 400× magnification and used for assessing the smooth muscle surface density. Ctrl: control group, composed of Wistar Kyoto rats (images A, E, and I); BPH: group of benign prostatic hyperplasia models (images B, F, and J); BPH + D: group of BPH models receiving dutasteride (images C, G, and K); BPH + DS: group of BPH models receiving dutasteride and sildenafil (images D, H, and L).
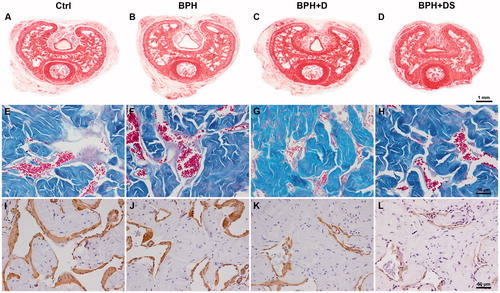
Table 2. Data of penile morphometric analyses.
The connective tissue Sv increased 16.7% in BPH, in comparison to Ctrl group. In BPH + D and BPH + DS groups, the connective tissue Sv was higher than BPH group, by 13.3% and 8.4%, respectively.
The sinusoid space Sv was reduced by 32.2% in BPH, in comparison to Ctrl group. This parameter was further reduced by 22.6% in BPH + D (). The elastic fibers Sv was similar among Ctrl and BPH groups, but presented an increasing of 51.6% and 65.6% in BPH + D and BPH + DS, when compared to the BPH group.
In regards to the smooth muscle Sv, although no difference was observed among Ctrl and BPH groups, treated animals from BPH + D and BPH + DS groups had a decrease of 28.6% and 21.4%, respectively, in comparison to BPH group ().
When analyzing the collagen type predominant in the CC, it was observed that all animals showed a type I (reddish appearance) collagen predominance, with no distinction among the groups.
Discussion
Erectile dysfunction is a frequent side effect associated with dutasteride (as well as other 5ARI) continuous use [Citation32]. One mechanism by which dutasteride prevents normal erection is by altering CC morphology. It has been shown that animals receiving dutasteride presents reduced smooth muscle and augmented connective tissue and elastic fibers in CC [Citation21], and these alterations are associated with reduced intracavernosal pressures [Citation33]. This study confirmed these penile modifications after dutasteride administration in a well-accepted BPH rodent model [Citation21,Citation23,Citation34]. Further, it was also pointed that sildenafil alone was effective for improving International Prostate Symptom Score as well as voiding symptoms in BPH/LUTS patients [Citation35].
The first line therapy for ED treatment are PDE5I, among which sildenafil was the first available and is still one of the most commonly used. For patients with 5ARI-associated ED, the use of PDE5I has been proposed and good clinical outcomes were reported [Citation14,Citation15]. Even though, it has not been evaluated before if the concomitant use of sildenafil could prevent the penile morphological alterations caused by dutasteride.
In this study, it was observed that most altered parameters in animals treated only with dutasteride (area of the CC without tunica albuginea, smooth muscle Sv, connective tissue Sv, and elastic fibers Sv) were similarly modified in animals receiving sildenafil and dutasteride. Thus, the 5ARI treatment was not effective in preventing the morphological alterations caused by dutasteride in the penile tissue.
As it is known, penile morphology (as well as in most of organs) is closely related to its function. The adequate proportion of cavernosal structures is of importance for normal erection, in both man [Citation36] and experimental animals [Citation30,Citation37,Citation38]. Thus, it seems that the erectile function improvement promoted by PDE5I in man under 5ARI treatment is due to its effects on nitric oxide synthesis instead of acting on penile morphology. As dutasteride has been implicated in the reduction of nitric oxide synthase [Citation39], the benefits of PDE5I may rely on this effect. This drug increases the synthesis of nitric oxide and generates relaxation of the endothelial smooth muscle of the CC, increasing blood flow in the sinusoid [Citation19,Citation20].
The CC is the pivotal morphological structure for penile erection. The structural composition of CC is basically smooth muscle fibers, connective tissue, vascular trabeculae, and blood vessels, each one with its own characteristics and functions. The cavernosal smooth muscle needs to relax, allowing the vascular trabeculae to be fulfilled with blood, raising the intracavernous pressure and thus, promoting and maintaining erection. Meanwhile, the connective tissue, which is very associated with tissue resistance, must permit (to a certain point) penile increase and elongation while restraining an exaggerated expansion during erection (keeping high intracavernous pressure) and, further, it must have resilience to restore normal anatomy in the detumescent estate [Citation38]. Thus, adequate amounts of all cavernosal components are required to reach normal erection and detumescence [Citation36].
A high amount of connective tissue and a reduced amount of smooth muscle fibers in the CC of animals receiving dutasteride was observed in this study. The modifications of these components are associated with penile fibrosis, and were seen in penises from patients with ED [Citation40]. Some experimental conditions, which are clinically associated with ED, also led to penile fibrosis as reported by several studies. Among them, we may cite chronic stress, aging, cavernous nerve damage, diabetes mellitus, and androgen deprivation [Citation25,Citation30,Citation38,Citation40]. The concomitant use of sildenafil was not efficient in preventing neither the reduction of smooth muscle nor the raise of connective tissue in the studied animals.
In this study, the proportional amount of elastic fibers was markedly augmented in the CC of both BPH + D and BPH + DS animals. Despite the importance of elastic system fibers in tissues submitted to stretching forces and/or size modifications [Citation41,Citation42], there are few studies that have investigated this component on CC and its relation with ED. Some studies showed a reduction of cavernosal elastic fibers potentially associated with ED, as in diabetes and chronic stress [Citation25,Citation43], while other papers observed an increase of this component associated with aging and priapism [Citation44,Citation45].
It is known that higher amounts of elastic system fibers are observed in tissues under repetitively stretching forces and modifications of this component may represent a loss of the tissue architecture and function, which is a pathological feature of a several inflammatory and degenerative conditions [Citation46]. Although the role of elastic fibers in penile erection should be further investigated, it is our opinion that any altered amount of the cavernosal structures should be observed closely as it may have an impact on normal physiological responses.
The results of this study should not be directly transposed to humans. The penises of humans and rats had different structural organization and this could be considered a limitation of this study. Nevertheless, the same structural components are present in both species CC, and these components respond very similarly when exposed to similar conditions [Citation22,Citation38,Citation42]. Further, ED is commonly a multifactorial condition, and in this study, only BPH and pharmacologic components were involved. Recently, the concomitant therapy of 5-ARI and alpha-blockers [Citation47,Citation48] or the use of antimuscarinics were advocated for treating BPH [Citation49–51]; however, the effects of this class of drugs on CC morphology has not been evaluated.
In conclusion, in our experimental model, the treatment with dutasteride promoted morphological modifications in the corpus cavernous of a BPH rodent model. Concomitant treatment with sildenafil did not prevent the morphological changes caused by dutasteride; on the contrary, this treatment also promoted a further increase in elastic fibers in the corpus cavernous. Further studies are warrant to explain the mechanisms by which PDE5I improves erection in patients with ED associated with 5ARI treatment.
Disclosure statement
The authors have no conflict of interest to disclosure.
Additional information
Funding
References
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin North Am. 2016;43:289–297.
- Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008;5:2917–2924.
- Alcaraz A, Carballido-Rodriguez J, Unda-Urzaiz M, et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: change over time in real-life practice according to treatment-the QUALIPROST study. Int Urol Nephrol. 2016;48:645–656.
- Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004;46:547–554.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803.
- Alcantara-Montero A, Brenes-Bermudez FJ. Finasteride or dutasteride for the pharmacological treatment for male lower urinary tract symptoms due to benign prostatic hyperplasia? Actas Urol Esp. 2016; 40:268–269.
- Shigehara K, Koh E, Sakamoto J, et al. Effects of dutasteride on lower urinary tract symptoms and general health in men with benign prostatic hypertroplasia and hypogonadism: a prospective study. Aging Male. 2014;17:51–56.
- Shigehara K, Miyagi T, Nakashima T, et al. Effects of dutasteride on lower urinary tract symptoms: a prospective analysis based on changes in testosterone/dihydrotestosterone levels and total prostatic volume reduction. Aging Male. 2016;19:128–133.
- Wada N, Hashizume K, Matsumoto S, et al. Dutasteride improves bone mineral density in male patients with lower urinary tract symptoms and prostatic enlargement: a preliminary study. Aging Male. 2016;19:12–14.
- Qian X, Yu G, Qian Y, et al. Efficacy of 5alpha-reductase inhibitors for patients with large benign prostatic hyperplasia (>80 mL) after transurethral resection of the prostate. Aging Male. 2015;18:238–243.
- Maeda T, Kikuchi E, Hasegawa M, et al. A prospective longitudinal survey of erectile function status in symptomatic benign prostatic hyperplasia patients treated with dutasteride. Aging Male. 2016;19:111–116.
- Traish A, Haider KS, Doros G, et al. Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction. Horm Mol Biol Clin Investig. 2017;30.
- Traish AM, Haider KS, Doros G, et al. Finasteride, not tamsulosin, increases severity of erectile dysfunction and decreases testosterone levels in men with benign prostatic hyperplasia. Horm Mol Biol Clin Investig. 2015;23:85–96.
- Roehrborn CG, Casabe A, Glina S, et al. Treatment satisfaction and clinically meaningful symptom improvement in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: secondary results from a 6-month, randomized, double-blind study comparing finasteride plus tadalafil with finasteride plus placebo. Int J Urol. 2015;22:582–587.
- Serati M, Andersson KE, Dmochowski R, et al. Systematic review of combination drug therapy for non-neurogenic lower urinary tract symptoms. Eur Urol. 2019;75:129–168.
- Watanabe D, Yamashita A, Miura K, et al. Effects on sexual function in Japanese patients with benign prostatic hyperplasia upon switching from combination therapy with alpha1 blocker and dutasteride to combination therapy with tadalafil and dutasteride. Aging Male. 2018;20:1–6.
- Scaglione F, Donde S, Hassan TA, et al. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin Ther. 2017;39:370–377.
- Leoni LA, Leite GS, Wichi RB, et al. Sildenafil: two decades of benefits or risks? Aging Male. 2013;16:85–91.
- Li H, Bai G, Zhang X, et al. Effects of two different dosages of sildenafil on patients with erectile dysfunction. Am J Mens Health. 2017;11:525–530.
- Palmerini CA, Zucchi A, Fioretti F, et al. Erectile dysfunction and NO: variations of NO penile blood levels before and after sildenafil treatment. Int J Impot Res. 2009;21:321–325.
- Da Silva MHA, Costa WS, Fj BS, et al. The corpus cavernosum after treatment with dutasteride or finasteride: a histomorphometric study in a benign prostatic hyperplasia rodent model. Asian J Androl. 2018;20:505–510.
- Felix-Patricio B, Medeiros JL, Jr., De Souza DB, et al. Penile histomorphometrical evaluation in hypertensive rats treated with sildenafil or enalapril alone or in combination: a comparison with normotensive and untreated hypertensive rats. J Sex Med. 2015;12:39–47.
- Lujan M, Ferruelo A, Paez A, et al. Prostate apoptosis after doxazosin treatment in the spontaneous hypertensive rat model. BJU Int. 2004;93:410–414.
- Wang D, Zha X, Nagase K, et al. Effects of the 5alpha-reductase inhibitor dutasteride on rat prostate alpha1A-adrenergic receptor and its mediated contractility. Urology. 2015;85:704 e9–704 e14.
- Ribeiro CT, Costa WS, Sampaio FJB, et al. Evaluation of the effects of chronic stress applied from the prepubertal to the adult stages or only during adulthood on penile morphology in rats. Stress. 2019;22:248–255.
- Sena A. D S M D, Vargas RA, Souza D. B D, et al. Morphometric study of the corpus cavernosum after anabolic androgenic steroid administration in pubertal and adult rats. Acta Cir Bras. 2015;30:478–483.
- Furriel A, Campos-Silva P, Silva PC, et al. Diets rich in saturated and polyunsaturated fatty acids induce morphological alterations in the rat ventral prostate. PLoS One. 2014;9:e102876.
- Vargas RA, Oliveira LP, Frankenfeld S, et al. The prostate after administration of anabolic androgenic steroids: a morphometrical study in rats. Int Braz J Urol. 2013;39:675–682.
- Felix-Patricio B, De Souza DB, Gregorio BM, et al. How to quantify penile corpus cavernosum structures with histomorphometry: comparison of two methods. Biomed Res Int. 2015;2015:832156.
- Miranda AF, Gallo CB, De Souza DB, et al. Effects of castration and late hormonal replacement in the structure of rat corpora cavernosa. J Androl. 2012;33:1224–1232.
- Montes GS. Structural biology of the fibres of the collagenous and elastic systems. Cell Biol Int. 1996;20:15–27.
- Corona G, Tirabassi G, Santi D, et al. Sexual dysfunction in subjects treated with inhibitors of 5α-reductase for benign prostatic hyperplasia: a comprehensive review and meta-analysis. Andrology. 2017;5:671–678.
- Kilic S, Kolukcu E, Erdemir F, et al. The effects of oral 5-alpha reductase inhibitors on penile intracavernosal pressures and penile morphology in rat model. Urol J. 2018;16:205–211.
- Oudot A, Oger S, Behr-Roussel D, et al. A new experimental rat model of erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: the testosterone-supplemented spontaneously hypertensive rat. BJU Int. 2012;110:1352–1358.
- Ko WJ, Han HH, Ham WS, et al. Daily use of sildenafil 50mg at night effectively ameliorates nocturia in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: an exploratory multicenter, double-blind, randomized, placebo-controlled study. Aging Male. 2017;20:81–88.
- Costa WS, Carrerete FB, Horta WG, et al. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006;97:567–569.
- Kovanecz I, Rivera S, Nolazco G, et al. Separate or combined treatments with daily sildenafil, molsidomine, or muscle-derived stem cells prevent erectile dysfunction in a rat model of cavernosal nerve damage. J Sex Med. 2012;9:2814–2826.
- de Souza DB, Silva D, Cortez CM, et al. Effects of chronic stress on penile corpus cavernosum of rats. J Androl. 2012;33:735–739.
- Pinsky MR, Gur S, Tracey AJ, et al. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med. 2011;8:3066–3074.
- El-Sakka AI, Yassin AA. Amelioration of penile fibrosis: myth or reality. J Androl. 2010;31:324–335.
- Maia RS, Babinski MA, Figueiredo MA, et al. Concentration of elastic system fibers in the corpus cavernosum, corpus spongiosum, and tunica albuginea in the rabbit penis. Int J Impot Res. 2006;18:121–125.
- Pinheiro AC, Costa WS, Cardoso LE, et al. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802–1806.
- Abidu-Figueiredo M, Ribeiro IC, Chagas MA, et al. The penis in diabetes: structural analysis of connective tissue and smooth muscle alterations in a rabbit model. BJU Int. 2011;108:400–404.
- Abidu-Figueiredo M, Costa WS, Chagas MA, et al. Age-related changes in the concentration of elastic fibers in different regions of the rabbit penis. Acta Cir Bras. 2013;28:378–384.
- Costa WS, Felix B, Cavalcanti AG, et al. Structural analysis of the corpora cavernosa in patients with ischaemic priapism. BJU Int. 2010;105:838–841.
- Lewis KG, Bercovitch L, Dill SW, et al. Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1–21.
- Favilla V, Russo GI, Privitera S, et al. Impact of combination therapy 5-alpha reductase inhibitors (5-ARI) plus alpha-blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: a systematic review with meta-analysis. Aging Male. 2016;19:175–181.
- Roehrborn CG, Manyak MJ, Palacios-Moreno JM, et al. A prospective randomised placebo-controlled study of the impact of dutasteride/tamsulosin combination therapy on sexual function domains in sexually active men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). BJU Int. 2018;121:647–658.
- Kosilov K, Kuzina I, Kuznetsov V, et al. Cognitive functions and health-related quality of life in men with benign prostatic hyperplasia and symptoms of overactive bladder when treated with a combination of tamsulosin and solifenacin in a higher dosage. Aging Male. 2018;21:121–129.
- Kosilov K, Loparev S, Ivanovskaya M, et al. Additional correction of OAB symptoms by two anti-muscarinics for men over 50 years old with residual symptoms of moderate prostatic obstruction after treatment with tamsulosin. Aging Male. 2015;18:44–48.
- Kosilov K, Loparev S, Kuzina I, et al. The effective tool for self-assessment of adherence to treatment in patients with benign prostatic obstruction and overactive bladder symptoms. Aging Male. 2017;20:39–44.
