Abstract
The present study aimed to investigate the frequency of deep venous thrombosis (DVT) among patients with obstructive sleep apnea syndrome (OSAS). Patients who referred the preliminary diagnosis of OSAS were included in this study. D-dimer levels of all patients were measured, and D-dimer (+) patients were evaluated by Doppler USG of the lower-extremity. Mean age of the patient group was 52 ± 12 years and 31.8% (76/239) were women. The rate of D-dimer positivity among severe-OSAS cases (15/85) was significantly higher compared to the rest (13/154) (17.6% and 8.4%, respectively; p = 0.034). The risk of D-dimer positivity was elevated by 2.3 folds in severe-OSAS cases (OR: 2,324, 95% confidence interval: 1.048–5.152). Among 28 D-dimer (+) cases, 4 (14.2%) had DVT as demonstrated by USI of the lower-extremity. All four cases with DVT had severe OSAS. D-dimer was positive in 17.6% (15/85) of all severe OSAS cases. DVT was diagnosed in 4.7% (4/85) of severe-OSAS cases. DVT frequency was 26.6% (4/15) in D-dimer (+) severe-OSAS. Findings of this study indicate that severe-OSAS can be a significant risk factor for DVT. Additionally, data obtained in this study underline the benefits of questioning severe-OSAS patients with respect to DVT symptoms, investigating D-dimer levels and evaluating D-dimer (+) severe-OSAS cases for DVT prophylaxis.
1. Introduction
Obstructive sleep apnea syndrome (OSAS) is the most common form of sleep breathing disorders and it is a syndrome characterized by repetitive partial or complete collapses of the upper airways during sleep [Citation1]. Its incidence in the Turkish population varies between 1–5% [Citation2].
There have been recent increased amount of reports regarding the relation between OSAS and clinical conditions such as cardiovascular, cerebrovascular, thromboembolism, orthopedic surgery, homeostasis, even erectile dysfunction and hormonal therapy [Citation3–17].
OSAS is known to be associated with cerebrovascular and cardiovascular morbidity and mortality [Citation3,Citation4]. Cardiovascular diseases develop as a result of a multifactorial process, incorporating large fluctuations in negative intrathoracic pressure, intermittent hypoxia and hypercapnia, increased activity of the sympathetic nervous system, vascular endothelial dysfunction, oxidative stress, systemic inflammation, excessive thrombocyte activation and metabolic dysregulation [Citation5]. In OSAS, cerebrovascular damage develops as hypoxic stress can trigger systemic inflammation and platelet activation [Citation6].
Mean yearly incidence of venous thromboembolism (VTE) varies between 23–269/100000. The rate of VTE-associated mortality in untreated cases is almost 25–30%, while it drops down to 2–8% in treated cases. Mortality generally has a linear relationship with malignancies, cardiopulmonary comorbidities and old age [Citation7].
Risk factors for VTE can be reviewed under two classes: genetic (antithrombin deficiency, protein C and protein S deficiency, factor XII deficiency, prothrombin 20210A mutation, factor V leiden mutation, hyperhomocysteinemia, anti-cardiolipin antibodies) and acquired (old age, immobilization, obesity, trauma, hip fractures, surgical procedures, smoking and the use of birth control pills, pregnancy, postpartum period, malignancy, heart failure and hyperviscosity syndromes) [Citation8].
Evidence suggests that OSAS is associated with increased plasma fibrinogen levels, enhanced platelet activation and decreased fibrinolytic capacity, as well as increased hypercoagulability [Citation9]. Recently, increasing number of studies suggested a casual relation between OSAS and VTE [Citation10]. On the other hand, the number of recent studies demonstrating a relation between OSAS and VTE is limited and most of those are case-control studies [Citation11]. Further studies are needed to establish a casual relation between OSAS and VTE.
This cross-sectional study aimed to investigate DVT frequency among OSAS patients and to find out whether OSAS represents a risk factor for DVT.
2. Materials and methods
2.1. Study design
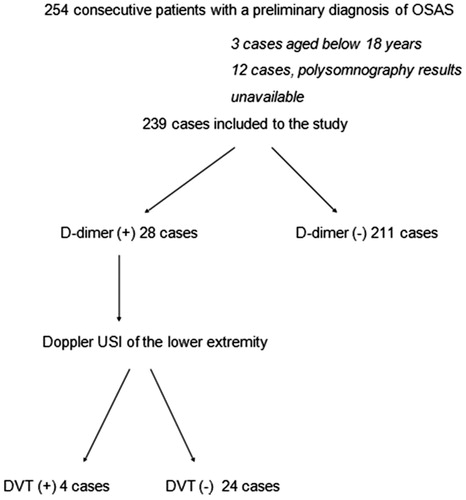
A total number of 254 consecutive patients, examined in the Sleep Breathing Disorders Laboratories of Duzce University Medical Faculty Pulmonology Department with a preliminary diagnosis of Obstructive Sleep Apnea Syndrome (OSAS) during a study period of one year, were evaluated in terms of the presence of DVT. Patients who did not consent to participate in the study, patients whose polysomnography results were not available, patients on anticoagulants, patients with a diagnosis of cancer, pregnant women, children, patients who were hospitalized within the last one month and patients with a known hematological disorder were excluded from the study. In total, 239 of 254 patients meeting eligibility criteria entered the study ().
2.2. Sleep assessments
All patients who had referred to sleep laboratories with a preliminary diagnosis of OSAS underwent full overnight polysomnography (Philips Respironics Model: Alice-5 PSG, Germany). Dual-channel EEG (electroencephalography), 2-channel EOG (electrooculography), 2-channel jaw EMG (electromyography), oral and nasal airflow (by thermistor and nasal cannula), thoracic and abdominal movements, body position, snoring, ECG and pulse-oximetry recordings were obtained (>6 h). All recordings were manually scored in digital medium. Apnea was defined as complete cessation of oral and nasal airflow for at least 10 s, and hypopnea was defined by more than 30% decrease in oral and nasal airflow for at least 10 s, accompanied by 3% desaturation or arousal. Patients with an apnea-hypopnea index (AHI) <5 were considered OSAS-negative, while those with AHI 5–15 were considered to have mild, AHI 15–30 to have moderate and AHI >30 to have severe OSAS.
2.3. Case assessments
D-dimer positivity was used as a screening test for DVT diagnosis. Cases were divided into two groups based on D-dimer positivity. (D-dimer levels were quantitatively measured by immunoturbidimetric methods using Roche COBAS 6000 Hitachi c501 device). Venous-system colored doppler ultrasonographic (USG) examination of the lower extremity was performed in D-dimer positive cases. Primary endpoint of the study was defined as a diagnosis of DVT in USI.
2.4. Statistical analysis
Statistical analyses were performed by SPSS 15.0 software. Chi-square test was used to compare D-dimer positivity between OSAS (+) and (−) cases, and also according to OSAS severity. Clinical numeric variables were compared between D-dimer (+) and (−) cases by student’s t-test. Pearson correlation analysis was performed to investigate the correlation between OSAS severity and D-dimer levels. p Values <0.05 were considered statistically significant.
3. Results
Mean age of the patients was 52 ± 12 years and 31.8% (76/239) of the study population were women.
Polysomnography investigations indicated that 85 (35.6%) cases had severe, 34 (14.2%) had moderate and 57 (23.8%) had mild OSAS, while 63 (26.4%) cases did not have OSAS.
Mean age, body mass index, creatinine levels, hemoglobin levels and hematocrit levels were not significantly different between D-dimer positive and negative cases. Mean AHI was significantly higher in D-dimer positive cases. The other polysomnographic parameters were not significantly different ().
Table 1. Comparison of D-dimer positive and D-dimer negative study groups in terms of clinical and polysomnographic findings.
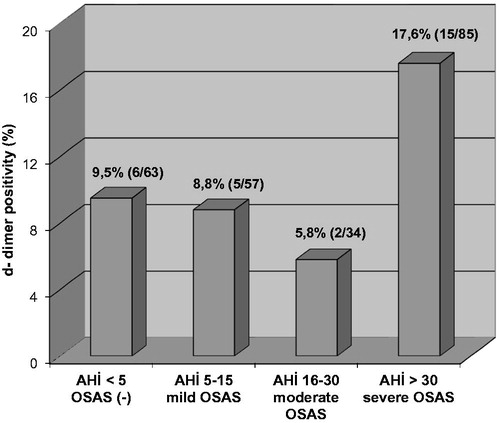
The rate of D-dimer positivity among all cases was 11.7% (28/239). D-dimer was found positive in 9.5% (6/63) of OSAS (−), 8.8% (5/57) of mild OSAS, 5.8% (2/34) of moderate OSAS, and 17.6% (15/85) of severe OSAS cases (). The rate of D-dimer positivity was significantly higher among severe OSAS cases (15/85) compared to the rest (13/154) (17.6% and 8.4%, respectively, p = 0.034). The risk of D-dimer positivity in severe OSAS was increased by 2.3 folds compared to all other cases (OR: 2.324, 95% confidence interval: 1.048–5.152).
Comparison of D-dimer positive and negative cases in terms of the presence and severity of OSAS did not indicate any significant difference, while the rate of D-dimer positivity was significantly elevated among severe OSAS cases compared to all other cases ().
Table 2. Comparison of D-dimer positive and negative cases in terms of gender, OSAS presence, and OSAS severity.
DVT was diagnosed by Doppler USI of the lower extremity in 4 of 28 D-dimer (+) cases (14.2%). All four cases with DVT had severe OSAS. D-dimer was positive in 17.6% (15/85) of all severe OSAS cases.
DVT was confirmed in 4.7% (4/85) of severe OSAS cases. DVT frequency in D-dimer (+) severe OSAS was estimated as 26.6% (4/15). Considering all 176 patients diagnosed with OSAS based on polysomnography, the rate of DVT was 2.2%.
4. Discussion
In the present study, the rate of D-dimer positivity was higher among cases diagnosed with severe OSAS compared to the rest of study population (17.6%). Moreover, all cases having a thrombus as demonstrated by Doppler USG were in the severe OSAS group. These findings suggest that severe OSAS seems to be a risk factor for DVT.
Studies investigating the relation between OSAS and DVT can be classified into two groups, as those “evaluating OSAS prevalence in patients with pulmonary embolism” and those “evaluating VTE prevalence in OSAS patients.”
In a previous study, Ambrosetti et al. monitored 89 patients with polysomnographically diagnosed OSAS, who used CPAP therapy and did not have a permanent or transient risk factor, for 3 years and reported that the rates of DVT and VTE development were 0.8% and 0.4%, respectively; rates that were higher than overall population [Citation18]. In the present study, 176 patients were diagnosed with OSAS based on polysomnography. According to cross-sectional evaluations, 4 of 176 patients had a thrombus, translating into a DVT development rate of 2.2%. Contrary to the study of Ambrosetti et al., which was limited to patients with moderate and severe OSAS, our study also included patients with mild OSAS, but the rate of DVT development was still higher. Preliminary assessment of DVT among asymptomatic patients by D-dimer screening in the present study can explain the higher rate of DVT diagnosis.
In their study comparing 5680 OSAS cases with 4505 OSAS-negative cases in terms of DVT, Chou et al. found that DVT was 3.11 folds more common among OSAS cases and concluded that OSAS can be an independent risk factor for DVT [Citation19]. In the present study, 2.3 folds risk elevation was only observed among severe OSAS cases.
Lin et al. followed 15.664 cases, 1424 of which had OSAS, for 5 years and demonstrated OSAS to be an independent risk factor for VTE and DVT [Citation20].
Peng et al. compared 3511 OSAS patients with 35110 controls in terms of DVT and/or PTE development and concluded that OSAS represents an independent risk factor for DVT and PTE [Citation21].
D’Apuzzo et al. evaluated PTE prevalence among 258.455 patients requiring total knee or total hip arthroplasty surgery, 16.608 of which had concomitant OSAS, and found OSAS to be an independent risk factor for PTE [Citation16].
In the present study, entire patient cohort was evaluated for OSAS by full PSG and DVT was diagnosed only among the cases with severe OSAS. Additionally, OSAS-negative patients evaluated in this study were not selected among healthy volunteers, but, they were patients representing with a clinical picture of OSAS, whose PSG ruled out OSAS diagnosis. We believe that such a control group represents a better match in terms of demographical characteristics and clinical status.
Kezban et al. evaluated OSAS in 30 patients diagnosed with PTE, and found that the frequency of OSAS was significantly higher among PTE patients without apparent risk factors compared to PTE patients with major risk factors. The authors argued that OSAS may represent a major risk factor for PTE [Citation22].
In the recent years, increasing number of clinical trials have been reporting significantly higher rates of OSAS among VTE patients compared to control groups [Citation17,Citation23–29].
In a study performed by Epstein et al., 270 cases with a preliminary diagnosis of PTE were evaluated and 71 cases with established PTE diagnosis constituted the study group, while 199 cases without PTE formed the control group. Demographical and clinical characteristics of all cases were assessed. Results from the multivariate analysis showed that PE was independently associated with risk of OSA [Citation23].
Arnulf et al. used PSG to evaluate OSAS in 68 VTE patients (10 DVT and 58 PTE) and reported a higher rate of VTE among OSAS patients [Citation24].
Sapala et al. assessed OSAS in 5554 patients who underwent bariatric surgery for morbid obesity, 12 of whom had PTE and reported a higher OSAS prevalence among patients experiencing fatal PTE [Citation25].
In another study, Mraovic et al. investigated OSAS in 7282 patients with planned total knee or hip arthroplasty. In that study, 107 patients had PTE and OSAS prevalence was not found to be significantly higher among PTE patients [Citation17].
Bosanquet et al. evaluated OSAS in 840 VTE patients and reported a higher OSAS prevalence among VTE patients compared to the general population [Citation26].
Arzt et al. compared sleep breathing disorders (SBD) between 82 patients with and 82 patients without VTE, and reported a higher rate of VTE among SBD patients as well as an increase in VTE frequency by increasing severity of SDB. Moreover, they diagnosed central sleep apnea syndrome in 5% of the cases and suggested that central sleep apnea syndrome could be an independent risk factor for VTE [Citation27].
Kosovali et al. compared 28 PTE patients with 45 controls and detected OSAS in 21% of the PTE group while none of the subjects in the control group had OSAS. The authors concluded that OSAS is an independent risk factor for PTE [Citation28].
Alonso-Fernandez et al. compared 107 PTE patients with 102 controls in their study and diagnosed OSAS in 9.8% of the patients in PTE group and 4.1% of individuals in the control group. They concluded that OSAS represents an independent risk factor for PTE [Citation29].
All above referred studies support a relation between OSAS and VTE. However, the risk factors, their severity and complications for OSAS involve several parameters interacting with each other in terms of DVT development. For instance, obesity is an important risk factor both for DVT and OSAS, and obesity also increases with increasing disease severity in severe OSAS. AHI take account only the number of apnea and hypopnea regardless of their duration and morphology. The mean obstructive apnea duration may also effect disease severity in addition to AHI [Citation30].
Similarly, several complications associated with disease severity in OSAS may effect DVT development. In the present study, all DVT cases were noted in severe OSAS patients, suggesting that DVT in the presence of OSAS can be associated with disease severity and/or complications. Contrary to the previous studies, the strength of this study was that it included a control group of clinically-matched real cases with PSG indication. Although concomitant diagnoses and medication use were not questioned in the study group, this limitation was minimized by selection of a control group among clinically-matched cases. This is further supported by the absence of a statistically significant difference in terms of age, gender and BMI between D dimer (+) and (−) cases.
Another limitation of the present study is that it included relatively low number of cases and was based on a cross-sectional analysis rather than a long-term follow-up. Still, contrary to the previous studies, this study took a step forward and investigated all cases, including the controls, by polysomnography. Moreover, after D-dimer screening and in the absence of clinical complaints, major complications such as pulmonary embolism were diagnosed by Doppler USI before they actually developed in subclinical DVT cases, which is the most significant strength of this study. Findings of this study are further substantiated by the use of a single Doppler USI to detect a high DVT prevalence in a cross-sectional investigation (4.7% (4/85) in severe OSAS, 26.6% (4/15) in D-dimer (+) severe OSAS).
We believe that the use of D-dimer test as a preliminary screening tool to assess such asymptomatic DVT cases can be elucidated in future clinical studies and in daily clinical practice.
Further clinical studies are required to demonstrate long-term outcomes and the effects of CPAP therapy on disease course in D-dimer (+) OSAS patients who do not have DVT based on Doppler USG.
5. Conclusions
Findings of the present study indicate that severe OSAS can be a significant risk factor for DVT. Moreover, data obtained in this study underline the benefits of questioning severe OSAS patients with respect to DVT symptoms, investigating D-dimer levels and evaluating D-dimer positive severe OSAS cases for DVT prophylaxis.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by Duzce University Medical Faculty Noninvasive Clinical Trials Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article
References
- The report of an American Academy of Sleep Medicine task force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689.
- Köktürk O. Obstructive sleep apnea syndrome; epidemiology. Tuberk Toraks. 1998;46: 193–101.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25.
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041.
- Turgut Celen Y, Peker Y. Cardiovascular consequences of sleep apnea: II-cardiovascular mechanisms. Anadolu Kardiyol Derg. 2010;10:168–175.
- Minoguchi K, Yokoe T, Tazaki T, et al. Silent brain infarction and platelet activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;175:612–617.
- Arseven O, Okumuş GN, Gül Öngen G, et al. Turkish thoracic society diagnosis and treatment report for pulmonary tromboembolism. Turk Thorac Soc. 2015;1–85.
- Konstantinides SV, Torbicki A, Agnelli G, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069.
- Von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest. 2003;124:1956–1967.
- Jiang X, Yongxiang W, Wei Z, et al. Higher dose of warfarin for patients with pulmonary embolism complicated by obstructive sleep apnea hypopnea syndrome. Heart Lung. 2014;43: 358–362.
- Lippi G, Mattiuzzi C, Franchini M. Sleep apnea and venous thromboembolism. A systematic review. Thromb Haemost. 2015;114:958–963.
- Shigehara K, Konaka H, Sugimoto K, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. 2018;21:99–105.
- Sengoren Dikis O, Acat M, Casim H, et al. The relationship of thiol/disulfide homeostasis in the etiology of patients with obstructive sleep apnea: a case-control study. Aging Male. 2019;3:1–8.
- Taken K, Ekin S, Arısoy A, et al. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male. 2016;19:102–105.
- Li X, Dong Z, Wan Y, et al. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a meta-analysis. Aging Male. 2010;13:82–86.
- D'Apuzzo MR, Browne JA. Obstructive sleep apnea as a risk factor for postoperative complications after revision joint arthroplasty. J Arthroplasty. 2012;27:95–98.
- Mraovic B, Hipszer BR, Epstein RH, et al. Preadmission hyperglycemia is an independent risk factor for in-hospital symptomatic pulmonary embolism after major orthopedic surgery. J Arthroplasty. 2010;25:64–70.
- Ambrosetti M, Lucioni A, Ageno W, et al. Is venous thromboembolism more frequent in patients with obstructive sleep apnea syndrome? J Thromb Haemost. 2004;2:1858–1860.
- Chou KT, Huang CC, Chen YM, et al. Sleep apnea and risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. Am J Med. 2012;125:374–380.
- Lin CC, Keller JJ, Kang JH, et al. Obstructive sleep apnea is associated with an increased risk of venous thromboembolism. J Vasc Surg Venous Lymphat Disord. 2013;1:139–145.
- Peng YH, Liao WC, Chung WS, et al. Association between obstructive sleep apnea and deep vein thrombosis/pulmonary embolism: a population-based retrospective cohort study. Thromb Res. 2014;134:340–345.
- Suner KO, Annakkaya AN, Toru U, et al. Is obstructive sleep apnea syndrome a risk factor for pulmonary thromboembolism? Chin Med J. 2012;125:3712–3718.
- Epstein MD, Segal LN, Ibrahim SM, et al. Snoring and the risk of obstructive sleep apnea in patients with pulmonary embolism. Sleep. 2010;33:1069–1074.
- Arnulf I, Merino-Andreu M, Perrier A, et al. Obstructive sleep apnea and venous thromboembolism. JAMA. 2002;287:2655–2656.
- Sapala JA, Wood MH, Schuhknecht MP, et al. Fatal pulmonary embolism after bariatric operations for morbid obesity: a 24-year retrospective analysis. Obes Surg. 2003;13:819–825.
- Bosanquet JP, Bade BC, Zia MF, et al. Patients with venous thromboembolism appear to have higher prevalence of obstructive sleep apnea than the general population. Clin Appl Thromb Hemost. 2011;17:E119–E124.
- Arzt M, Luigart R, Schum C, et al. “Circulation and Sleep” working group of the German Society of Sleep Research and Sleep Medicine (DGSM). Sleep-disordered breathing in deep vein thrombosis and acute pulmonary embolism. Eur Respir J. 2012;40:919–924.
- Kosovalı D, Uyar M, Elbek O, et al. Obstructive sleep apnea is prevalent in patients with pulmonary embolism. Clin Invest Med. 2013;36:E277–E281.
- Alonso-Fernández A, de la Peña M, Romero D, et al. Association between obstructive sleep apnea and pulmonary embolism. Mayo Clin Proc. 2013;88:579–587.
- Yılmaz Durmaz D, Güneş A. Which is more important: the number or duration of respiratory events to determine the severity of obstructive sleep apnea? Aging Male. 2019:1–6. doi:10.1080/13685538.2019.1630062