Abstract
Objectives
Obstructive Sleep apne syndrome is a disease with high morbidity and mortality. The aim of this study was to investigate the conditions affecting the mortality of patients diagnosed with OSAS at six year follow up.
Methods
970 patients who admitted to Sleep laboratory between 2011–2013 were evaluated retrospectively. 74 patients whose mortality data could not be accessed through the system were excluded. The patients who died until April 2019 were compared with the surviving group in terms of demographic, clinical, comorbidities and polysomnographic findings.
Results
Total 47 patients who died were older, had higher BMI, AHI and ODI values, lower minimum oxygen saturations compared with the survival group (p < .001). In the Cox-hazard regression analysis, BMI (hazard ratio (HR), 1.08; 95% CI, 1.04–1.12), age (1.12, 1.08–1.15), accompanying COPD (2.19, 1.08–4.43), accompanying CAD (2.76, 1.34–5.67) and AHI of >50/h (2.19, 1.19–1.4.05) were reported.
Conclusion
This study showed that OSAS increases the risk of death accompanied by CAD and COPD. It has also been shown that patients with higher AHI (AHI > 50/h) values die more. Therefore, it may be useful to classify the AHI> 50/h group as very severe OSAS instead of severe OSAS.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a clinical condition characterized by recurrent partial or complete collapse in the upper airways, commonly accompanied by oxygen desaturation [Citation1], affecting a significant part of the population with high mortality and morbidity.
Basic pathogenic mechanisms considered in the development of especially cardiovascular complications in OSAS are intermittent hypoxia, intrathoracic pressure fluctuations, and sleep disruptions thus developing sympathetic activation, oxidative stress, systemic inflammation, and metabolic dysregulation leading toendothelial damage [Citation2,Citation3]. Thiol/disulfide homeostasis is one of the important predictors of oxidative stress in recent years and impaired thiol/disulfide balance may play a role in the pathogenesis of OSAS [Citation4].
All organ systems are affected by similar pathogenetic mechanisms. OSA has significant effects on the entire endocrine system including reproductive system which is associated with hypogonadotropic hypogonadism due to altered gonadotropin synthesis and release [Citation5]. It is known that men with severe hypogonodism without OSAS have severe sleep disturbance and improved with hormone replacement therapy [Citation6]. Also, erectile dysfunction are frequently observed in OSA patients [Citation7, Citation8].
The most prevalent cardiovascular complications accompanying OSAS are hypertension, coronary artery disease, heart failure, or cardiac arrhythmia [Citation9,Citation10]. OSAS has been accepted, although with low level of evidence, as a risk factor for long-term poor clinical outcome in patientswith hypertension, obesity, diabetes, cardiovascular disease, and stroke [Citation11].
The gold standard diagnostic method of OSAS is full-night polysomnography [Citation12]. Apnea–hypopnea index (AHI) provides information regarding the disease severity, and the disease can be classified as mild, moderate, or severe according to AHI. Although AHI is not always correlated with the clinical presentation, severity, clinical course, probability of complication development of the disease, it is still accepted as the most commonly used indicator in clinical practice [Citation13].
Although the severity of the OSAS has been classified according to the AHI; the relationship between AHI, long-term results of the disease, morbidity and mortality statuses have not been completely elucidated. The aim of the present study is to evaluate the 6-year follow-up results in terms of mortality in OSAS patients between 2011 and 2013 and who were been recommended positive airway pressure (PAP) treatment along with the other treatments according to the disease severity, clinical, demographic, and polysomnographic results of the exitus group by comparing them with those of the survival group.
Methods
Patients
A total of 970 patients who had polysomnography performed in the sleep laboratory of the Süreyyapaşa Chest Diseases Hospital between 2011 and 2013 were included. Gender, age, Epworth sleepiness scale, accompanying additional diseases, and polysomnographic diagnosis of the patients were analyzed retrospectively. The study was conducted in accordance with the Declaration of Helsinki and approved by Süreyyapaşa Chest Diseases Training Hospital Local Ethics Committee.
A total of 970 patients were scanned from the date of admission to the first hospital until April 2019 from the Death Notification system. This program had been developed by the Ministry of Health in Turkey in order to quick and completely compile death statistics; thus providing each death case can be filled by the physician and recorded in the system, as alive or dead. Total 74 patients whose mortality data could not be accessed through the system were excluded. The clinical, anthropometric, and polysomnographic findings of the remaining 896 patients (633 male (71%) and 263 female (29%)) were analyzed.
Polysomnography
Polysomnography recordings for all patients were performed full-night and during the spontaneous sleep, using the GRASS brand polysomnography device, in the sleep laboratory. Electroencephalography, electro-oculography, chin and tibial electromyography, and electrocardiography were recorded (Grass-Telefactor Cephalo, An Astro-med Inc. Product Group, 2005, USA). Airflow and respiration effort were measured using nasal-oral “thermistor” and thoracoabdominal “piezoelectric” belts, respectively. The positions of the patients during sleep were recorded using the body position sensor. Audio and video recordings were taken full-night using a video camera. More than 10 s of pause in the air flow despite the continuing respiratory effort was scored as obstructive apnea, whereas more than 50% decrease in the airflow, which caused ≥3% oxyhemoglobin desaturation, was scored as hypopnea. AHI was determined by calculating the average apnea and hypopnea per hour throughout the sleep duration [Citation14].
Statistical analysis
Statistical Package for Social Sciences (SPSS) for Windows 22.0 software was used to perform the statistical analysis of the data. Kolmogorov- Smirnov normality test was used to show the normal distribution of numerical values. Mean ± standard deviation (SD) was used for parametric continuous variables. Differences between the groups were analyzed using Student's t tests for parametric continuous variables. Chi-square test was used for bivariate variables. P values less than 0.05 were considered statistically significant. Cox regression analysis was used for risk hazard ratio for mortality. Age, BMI, Coronary arterial disease, COPD and AHI > 50/hour were included in the Cox regression model. Kaplan Meier survival analysis was performed.
Results
The average age of 896 patients was 50 ± 11 years and BMI was 32.5 ± 6.3 kg/m2. According to the diagnosis groups, 84,184, 215,and 413 (9.4%,20.5%, 24%, and 46.1%) patients had AHI of < 5/h, 5–15/h (mild OSAS),15–30/hours (moderate OSAS), and > 30/hour (severe OSAS), respectively. Regarding the treatment analysis, 54% patients were prescribed nasal continuous PAP (CPAP), 20.6% were bilevel PAP (BPAP), 4.4% patients had additional Oxygen added to the PAP treatment, and 3.5% rejected the treatment (). During the 6-year follow-up, total 47 patients who died were older, had higher BMI (body mass index), AHI and ODI values, lower minimum oxygen saturations compared with the survival group, and these data were highly significant (p < .001; ). In the exitus group, accompanying HT, DM, CHF, CAD, and COPD were prevalent (p < .001; ). During 6 years of follow-up, 36 (5.6%) of 633 male patients and 11 (4.1%) of 263 female patients died (p = .22).The duration from diagnosis to death was 1533 days for men and 1614 for women. According to the AHI group, AHI <5 was in 84 patients and only one patient was exitus in this group, whereas the most exitus were seen in the group with AHI of >30. There was no significant difference between the exitus and survival groups in terms of snoring, witnessed apnea, and daytime sleepiness. Death rate was significantly higher especially in the groups that discontinued the treatment and in those who were given BPAP and BPAP + Oxygen (p < 0.001; ).
Table 1. Demographic, clinical and polysomnographic data of patients.
Table 2. Comparison of demographic, polysomnographic and co-morbidities of the exitus and surviving groups.
Table 3. Comparision of the exitus and surviving groups according to gender, clinical, disease severity and treatment options.
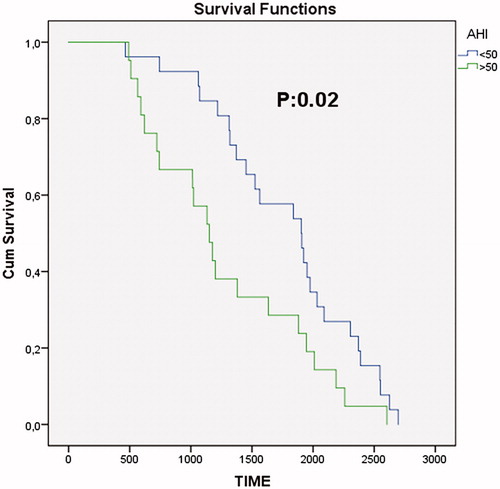
Particularly, when the patients were categorized into new two groups as less and more than AHI of 50/h, a high mortality rate has been occured in the group with AHI of >50 (). In the Cox-hazard regression analysis, BMI [hazard ratio (HR),1.08; 95% CI, 1.04–1.12], age (1.12, 1.08–1.15), accompanying COPD (2.19, 1.08–4.43), accompanying CAD (2.76, 1.34–5.67) and AHI of >50/h (2.19, 1.19–1.4.05) were reported ().
Table 4. According to regression analysis, the cases with the most significant relationship with mortality.
Discussion
In the 6-year follow-up of OSAS patients and those who received PAP treatment in addition to other treatment recommendations, mortality was remarkably high among those with AHI of >50/hour, with CAD and COPD comorbidities. Moreover, the number of patients given BPAP and those who required additional Oxygen support was more than those in the exitus group.
There are several studies on the relationship between AHI and OSAS mortality. In the study by He et al. on mortality and apnea index, it was shown that mortality increases at an apnea index of >20. However, the case group of this study was small, and the follow-up duration was quite short [Citation15]. Furthermore, Yaggi et al. in their 3.5-year follow-up showed that mortality increased twice among the patients with an average AHI of 35/hour compared with that in patients in the non-apneic group [Citation16]. Young et al. found that in the Wisconsin sleep cohort study, the HR for all-cause mortality in the AHI >30/h group with an 18-year follow-up period is 3.2 [Citation17]. Marshall et al. showed that AHI of >15 is an independent risk factor for all-cause mortality among patients with OSAS followed up for 20 years [Citation18]. In the present study, similar to the previous ones, mortality rate was higher in the AHI >30/hour group compared with that in the other groups. Additionally, the mortality risk significantly increased especially in the AHI >50/hour group (HR,2.19; 95% CI, 1.19–1.4.05). As an interesting and recent report, apnea-hypopnea duration index is recommended instead of AHI in OSA severity classification [Citation19]. According to our results, the mortality risk in patients with AHI of >50/h at the time of diagnosis was high; therefore, we believe that close monitoring of these patients can be useful. Age, male gender, and BMI can be considered as the risk factors for OSAS [Citation20]. However there was no similar risk for mortality [Citation17]. In our study, the exitus patients were older and more overweight, but there was no apparent increase in the mortality risk (BMI:HR,1.08; 95% CI, 1.04–1.12;age:HR,1.12; 95% CI, 1.08–1.15). No difference was observed in terms of gender.
OSAS is an important disease that can negatively affect all organ systems in the body. The development of excessive local and systemic inflammation leads to complications in all organ systems, particularly the cardiovascular one [Citation21]. Intermittent hypoxemia, acidosis, increased sympathetic activation, and accompanying changes in intrathoracic and cardiac transmural pressures caused by obstructive apnea are considered as potential triggers for cardiac ischemia. In the long term, endothelial dysfunction and systemic inflammation may lead to CAD thus assisting in explaining the pathogenesis of cardiac and vascular diseases [Citation22]. In the present study, in the exitus group, the number of patients with HT, DM, CAD, CHF, and COPD were significantly higher than those in the survival group. In a study about mortality in OSAS, cardiovascular complications had found to be the most important risk factor for mortality [Citation23]. Young et al. reported that after excluding patients using CPAP, there was a significant increase in cardiovascular mortality with severe OSAS [adjusted HR (95% CI), 5.2 (1.4, 19.2)] [Citation17]. In the Busselton Health study cohort, severe OSAS resulted in increased risk for all-cause mortality, whereas the same risk was not found for cardiovascular mortality [Citation18]. In the present study, cardiovascular complications accompanying HT, CAD, and heart failure were significantly higher in the mortality group than those in the survival group. In Cox regression analysis, mortality risk related with CAD increased, which is consistent with previous studies (HR, 2.76; 95% CI, 1.34–5.67) [Citation17,Citation23].
COPD is one of the most important causes of mortality [Citation24]. In COPD, chronic hypoxemia and systemic inflammation are observed. Intermittent hypoxia observed in OSAS worsens the situation in chronic hypoxia [Citation25]. Although the mechanism cannot be completely explained, mortality risk increases with OSAS and COPD comorbidity [Citation26,Citation27]. In the present study, it was found that OSAS patients with COPD, mortality rate was twice than OSAS patients without COPD (HR, 2.19; 95% CI, 1.08–4.43).
The most effective and reliable treatment method in OSAS is PAP treatment [Citation28]. CPAP treatment is also used in the treatment. Moreover, BPAP is indicated in COPD, restrictive pulmonary disease, or hyperventilation cases and when higher pressure is needed. Despite PAP treatment, if hypoxia continues, addition of external Oxygen is required [Citation29]. PAP treatment is proven to decrease the complications occurring due to OSAS; however, its effect on mortality is still unclear [Citation23]. In the present study, patients given BPAP had more exitus compared with those in the survival group. This can be explained with the mortality risk being higher with COPD comorbidity. Similarly, mortality was more in the group that was provided additional Oxygen treatment.
There are some limitations of the study. We were unable to analyze the daily use of PAP treatment by patients because our patient population belonged to the low-income group; therefore standard PAP devices of low quality were used, which were paid by the our social security system and which did not include the software properties that measured the duration of device usage by the patients, such as a memory card. In this 6-year follow-up study, we do not know whether there were other newly developing additional diseases, which may have contributed to the mortality in this patient group.
In conclusion, despite some limitations, we found that the disease course led to high mortality in patients with CAD, COPD additional disease status, high AHI values in polysomnography (AHI >50/h), those given BPAP in the first titration night, and those with additional Oxygen treatment. Therefore, we think that close monitoring and frequent follow-up of these patients might be helpful. Moreover, considering our 896 case OSAS cohort data, we believe that the OSAS severity classification system ifprevalently used according to the AHI, it could be beneficial to classify, especially the AHI >50/h group, as very severe OSAS instead of severe OSAS.
Therefore, we believe that including more patients, well-designed, prospective, multicenter studies are needed to identify possible causes of death in OSAS patients.
Authors’ contributions
Literature research (GCA, AOG)
Data Collection (GCA, OS, SS)
Study Design (GCA, OS, TY)
Analysis of Data (GCA, CS)
Manuscript Preparation (GCA, TY)
Review of Manuscript (All authors)
Disclosure statement
Authors report no conflict of interests related to the current study.
References
- American Academy of Sleep Medicine. ICSD-3: The international classification of sleep disorders, 3rd ed. Darien, IL: AASM, 2014.
- Epstein LJ, Kristo D, Strollo PJ. Clinical guideline for the evaluation, management and long-term care of obstructive Sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276.
- Chami HA, Fontes JD, Vasan RS, et al. Vascular inflammation and Sleep disordered breathing in a community-based cohort. Sleep. 2013;36(5):763–768C.
- Sengoren Dikis O, Acat M, Casim H, et al. The relationship of thiol/disulfide homeostasis in the etiology of patients with obstructive sleep apnea: a case-control study. Aging Male. 2019;3:1–8.
- Lanfranco F, Motta G, Minetto M, et al. Neuroendocrine alterations in obese patients with sleep apnea syndrome. Int J Endocrinol. 2010;2010:474518.
- Shigehara K, Konaka H, Sugimoto K, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. 2018;21(2):99–105.
- Taken K, Ekin S, Arısoy A, et al. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male. 2016;19(2):102–105.
- Li X, Dong Z, Wan Y, et al. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a meta-analysis. Aging Male. 2010;13(2):82–86.
- Peppard PE, Young T, Palta M, et al. Prospectivestudy of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384.
- Baguet JP, Barone-Rochette G, Tamisier R, et al. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012;9(12):679–688.
- Mansukhani MP, Bellolio MF, Kolla BP, et al. Worse outcome after stroke in patients with obstructive sleep apnea: an observational cohort study. J Stroke Cerebrovasc Dis. 2011;20(5):401–405.
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235.
- Kendzerska T, Mollayeva T, Gershon AS, et al. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev. 2014;18(1):49–59. 8.
- Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131.
- He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94(1):9–14.
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008; 31(8):1071–1078.
- Marshall NS, Wong KKH, Cullen SRJ, et al. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study Cohort. J Clin Sleep Med. 2014;10(4):355–362.
- Yılmaz Durmaz D, Güneş A. Which is more important: the number or duration of respiratory events to determine the severity of obstructive sleep apnea? Aging Male. 2019;26:1–6.
- Starling JR. Sleep related breathing disorders. Obstructive sleep apnea: definitions, epidemiology, and natural history. Thorax. 1995;50:683–689.
- Hatipoğlu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hyptothesis. Respiration. 2003;70(6):665–671.
- Somers VK, White DP, Amin R, American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Circulation. 2008;118(10):1080–1111.
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. . 2005;365(9464):1046–1053.
- The Centers for Disease Control and Prevention (CDC). CDC Wide-ranging Online Data for Epidemiologic Research (WONDER) Online Database: Compressed mortality file 1999- 2015; ICD-10 Codes: J40-J44. CDC website. Available from: http://wonder.cdc.gov/cmf-icd10.html 2016. Accessed May 2018.
- Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661.
- Stanchina ML, Welicky LM, Donat W, et al. Impact of CPAP use and age on mortality in patients with combined COPD and obstructive sleep apnea: the overlap syndrome. J Clin Sleep Med. 2013;9(8):767–772.
- Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331.
- Grunstein R, Sullivan C. Continuous positive airway pressure for sleep breathing disorders In: Kryger MH, Roth T, Dement WC (eds). Principles and practice of sleep medicine. Philadelphia: WB Saunders Company, 2000. pp. 894–912.
- Kushida CA, Littner MR, Hirshkowitz M, American Academy of Sleep Medicine, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380.