Abstract
Background and objectives
The aim of this study was to investigate the contractile effects of oxytocin (OT) in human detrusor muscle in in vitro conditions.
Material and Methods
Human detrusor muscle samples were obtained from seven patients that undergone radical cystectomy. Four female Wistar rats’ uterine samples were used as control. Contractile responses were tested of carbachol in organ bath. Cumulative concentration response curves were constructed to OT and then the strips were incubated with atosiban (OT antagonist) and a second concentration response curve to OT were constructed.
Results
Carbachol, contracted all human strips for the functionality test whereas OT in any concentrations did not produce significant contraction on all human strips. In only one bladder strip and in a very high concentration slight contraction was recorded. Moreover no contractile response was recorded in any OT concentrations in the presence of atosiban. The rat uterine strips responded to OT in a dose dependent manner. Atosiban, the OT receptor antagonist diminished totally those contractile responses.
Conclusion
It is been demonstrated here that there is no contractile response to OT in human detrusor muscle. These findings should be supported by further investigations determining the presence of the OT receptor in human detrusor.
Introduction
A neurohypophyseal hormone, oxytocin (OT) is a nonapeptide which acts as a neurohormone, neurotransmitter, or a neuromodulator. OT is synthesized in supraoptic nucleus and paraventricular nucleus of hypothalamus. The supraoptic nucleus projects axonal fibers to the posterior pituitary gland where OT is secreted into bloodstream and the paraventricular nucleus projects to extrahypothalamic brain areas and releases OT into the cerebrospinal fluid, respectively [Citation1,Citation2].
Previous studies demonstrated that OT exerts a wide spectrum of central and peripheral effects [Citation1,Citation2]. The expression of OT and its receptor has now been identified in a variety of peripheral tissues [Citation1,Citation3–5]. In addition to systemically circulating OT, the peptide is also produced and secreted locally in some tissues such as testes, epididymis, and prostate [Citation3,Citation4]. It has also contractile activities on a various smooth muscles such as prostate, corpus cavernosum and tunica albuginea of testes [Citation6–8].
It was previously demonstrated in both in vitro and in vivo studies that OT induces intrinsic contractile activity on detrusor smooth muscles of different species such as rabbit and rat [Citation9,Citation10]. Although there were clear affinity between bladder smooth muscle tissue and OT as shown in those studies to our knowledge, no human studies related on this topic was performed up to day [Citation9,Citation10]. In addition, a mini-review about the role of oxytocin in the development of overactive bladder syndrome has been published recently [Citation11]. In this review, it has been suggested that OT may contribute to the development of overactive bladder (OAB) through prostaglandins, estrogens or dopaminergic central or peripheral pathways.
The aim of this study was to investigate the possible contractile effects of OT in human detrusor smooth muscle tissue with special emphasis on further therapeutic potential of OT in underactive bladder or its antagonists in OAB.
Material and methods
Human subjects
Human detrusor samples were obtained from seven patients (3 female and 4 male; mean age 63 years, range 28–80) that undergone radical cystectomy operation with the diagnosis of muscle invasive bladder cancer. It was conducted in accordance with the Declaration of Helsinki. The present study protocol were reviewed and approved by the Institutional Review Board of Kartal Training and Research Hospital (Approval No. 15.08.2012/14). A written informed consent to participate in this study was obtained from each patient before enrollment. Specimens were not obtained from patients meeting the following exclusion criteria: use of narcotic analgesics; impaired liver or kidney function (creatinine >2mg/dl); presence of discrete anatomical urinary tract abnormalities (e.g. urethral strictures, bladder diverticula or fistula); presence of a known neurological disorder (multiple sclerosis, Parkinson’s disease, spina bifida or spinal cord injury/trauma); diabetes mellitus; a history of previous malignancy and/or treatment with chemotherapy/radiotherapy. Tissue samples were taken perioperatively from the macroscopically non-tumor infiltrating bladder area (visually normal bladder) at cystectomy as soon as the whole bladder was excised. All detrusor specimens (5 × 2 cm) were harvested from the dome of the bladder.
Preparation of rat uterine smooth muscle samples
Uterine samples were obtained from 4 female Wistar rats weighing between 250–350 gr were sacrificed by decapitation under ketamine (100 mg/kg, IP) and xylazine (10 mg/kg, IP) anesthesia. The abdomen was accessed through a lower midline incision and then the symphysis was opened. The uterus was carefully dissected free from the pelvic wall, and immediately placed in ice-cold Krebs solution (composition detailed below). Uterine smooth muscle preparations were dissected and the longitudinal strips were transferred to 20 ml tissue baths containing Krebs solution. The procedures used and the care of animals were approved by the Institutional Review Board of Kartal Training and Research Hospital (Approval No. 15.08.2012/14).
Experimental design
Preparation of human detrusor samples
All human tissue samples were immediately placed in pre-oxygenated ice-cold Krebs solution after resected in the operation room, and kept at +4 °C during transportation to in vitro organ bath laboratory. Longitudinal strips of human urinary bladders were prepared as described above for rat uterine strips and mounted in an organ bath containing 20 ml Krebs solution. Two longitudinal strips from each human bladder tissue were prepared and studied simultaneously as described above for rat uterine strips and mounted in an organ bath containing 20 ml Krebs solution.
In vitro organ bath experiments
Krebs solution (composition in mMol l−1: NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgSO4, 1.2; NaHCO3, 25.0; KH2PO4, 1.2; glucose, 11.1) at +37 °C bubbled with a mixture of 95% O2 and 5% CO2 under a resting tension of 1.0 g and were allowed to equilibrate for 1 h. Isometric contractions were then recorded on a polygraph (Model 7; Grass Instruments, Quincy, MA, USA) via a force displacement transducer (FT03; Grass Instruments, Quincy, MA, USA). First of all, contractile responses were tested in 10−5 M final concentration of carbachol in organ bath in order to ensure the functionality of the strips’. After washing with Krebs solution several times and reaching the pre-contraction level, cumulative concentration response curves were constructed to OT, using incremental increases in concentration spaced at 0.5 log intervals (between 10−9 M to 10−5 M final concentrations). Concentrations were added once a sustained response to the previous concentration was reached. An interval of 45 min was then allowed during which the tissues were washed with Krebs solution several times and reaching the pre-contraction level. After OT concentration-response records were taken the strips were incubated with atosiban -the OT antagonist- 10−5 M for 15 min and a second concentration response curve to OT were constructed for any possible effect over the OT effect on human bladder smooth muscle tissue. Experiments of human and rat strips were conducted simultaneously on the same day.
Data analysis
The individual maximal contractile responses of the urinary bladder strips were accepted as 100% and EC50 values and the slopes of concentration-response curves were calculated by means of the logistic transformation with individual maximal responses to 10−5 M carbachol accepted as 100%, and linear regression analysis. The EC50 values of OT were compared for significance by Student's t-test. The level of significance was accepted as p < 0.05.
Drugs
Oxytocin was purchased from Deva Pharmaceutical Company, carbachol was purchased from Sigma, and atosiban was purchased from Ferring Pharmaceutical Company.
Results
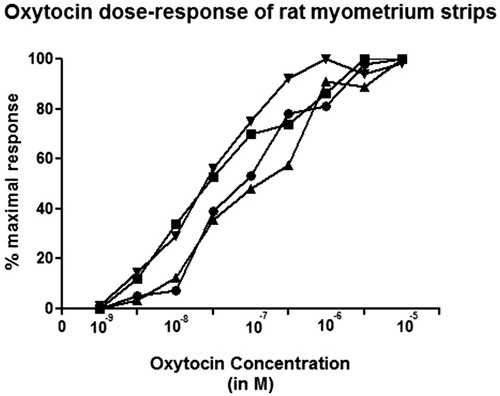
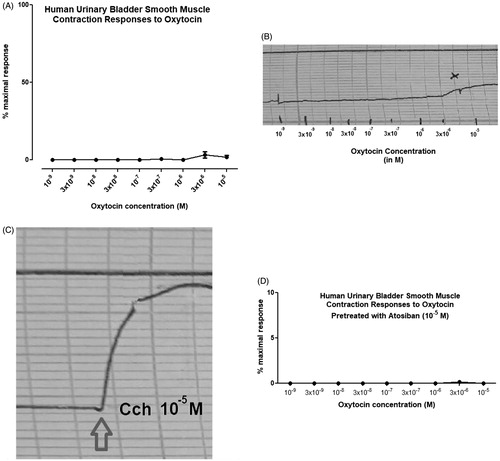
Carbachol, the muscarinic receptor agonist contracted all human bladder smooth muscle strips in a final concentration of 10−5 M for the functionality test () whereas OT in any concentrations between 10−9 M to 10−5 M did not produce significant contraction on all human urinary bladder smooth muscle strips (). In only one bladder strip (male 60 years old) and in a very high (3 × 10−6 M) concentration slight contraction was recorded (). Moreover no contractile response was recorded in any OT concentrations between 10−9 M to 10−5 M in the presence of atosiban, the OT receptor antagonist (). As no responses to OT in all human bladder smooth muscle strips were recorded we decided to test whether OT is active or not. For this purpose OT was tested in rat uterine smooth muscle at in vitro organ bath. The rat uterine smooth muscle strips responded to OT in a dose dependent manner (concentrations between 10−9 M to 10−5 M) (). Atosiban, the OT receptor antagonist diminished totally those contractile responses in a concentration of 10−5 M (data not given) in rat uterine smooth muscle strips. ( and ).
Figure 1. The contractile responses of the human urinary bladder smooth muscle strips to oxytocin without (a, b) or with atosiban (d) and carbachol (c) in isolated in-vitro organ bath. A- Cumulative oxytocin concentration-response curves for all human urinary bladder strips. B- Cumulative concentration response of patient ID: 2 (the only responsive tissue) in 10−6 M concentration. C- Carbachol concentration-response to 10−5 M (functionality test). D- Cumulative concentration response of the human urinary bladder smooth muscle strips to oxytocin pretreated with 10-5 M atosiban (Y axis is 10%).
Discussion
OT is expressed in a variety of tissues, as are its receptors. OT acts as a paracrine and/or autocrine mediator of various multiple biological effects in vivo. These effects are exerted primarily through interactions with G-protein coupled OT/vasopressin receptors which stimulate phospholipase C-mediated hydrolysis of phosphoinosides [Citation2,Citation12].
However presence of OT in human urine bladder and effects of OT on urine bladder were not investigated hitherto. It was shown in two previously performed studies that OT have effects on urine bladder in some animal species [Citation9,Citation10]. Romine et al. [Citation9] show that OT exhibits high intrinsic contractile activity on isolated strips of detrusor muscle from male New Zealand White rabbits. The responses to OT were partially inhibited by indomethacin. In this study evidence for OT receptors in the urinary bladder of rabbits through isotherm binding experiments have been presented. Thereafter, Pandita et al. [Citation10] observed that intrathecal OT stimulated bladder activity, causing a significant increase in micturition pressure, decrease in bladder capacity and micturition volume in conscious female rats undergoing continuous cystometry. When the antagonist or the nitric oxide synthase inhibitor was given intrathecally prior to intrathecal OT, the effects of OT were abolished. Additionally, in isolated detrusor strips, OT caused a concentration-dependent contraction; the concentration response curve was concentration dependently shifted to the right by the OT antagonist. Intra-arterial OT did not produce any change in the cystometric parameters. They concluded that the peripheral effect of OT was not responsible for the stimulation of bladder activity, since intra-arterial injection of OT had no effect and the induced bladder activity was a spinal effect [Citation10].
Additionally, Black et al. [Citation13] shown that stress induced bladder hypersensitivity was attenuated by the intraperitoneal administration of OT in female rats. Engle et al. [Citation14] also revealed that the intrathecal administration of an OT cause to abolishing of visceromotor reflexes to urinary bladder distension by inhibition of spinal dorsal horn neurons. In the scope of these studies, we concluded that central actions of oxytocin rather than peripheral effects are more important on the bladder functions.
Nevertheless these findings were not supported by following studies neither on human nor any other species. To our knowledge, this is the first human study with OT on human urinary bladder strips. Although in any bladder tissue samples of any patients in the study population we recorded contractile responses except for one slight (10% of maximal) contraction in the very high concentration (10−6 M) compared with the same tissue’s response to carbachol (). Also the statistical analysis showed no significant contraction between the studied OT concentrations (10−9 and 10−5 M). Following these findings in order to check the activity of OT, we tested the same concentrations in rat uterine strips and found the contractile responses as expected (). All together here we demonstrate that OT has no contractile effects on human urinary bladder smooth muscle tissue, although two animal studies were shown contractile effects of OT on detrusor muscles of different species. However, the results of animal studies cannot always be extrapolated to humans.
One of the primary aims of this study was to find a therapeutic option of OT in underactive bladder or its antagonists in OAB. Unfortunately we could not demonstrate the efficacy of neither OT nor atosiban in human urinary bladder detrusor muscle to be further utilization of these medications in human urinary bladder pathologies.
The major limitation of this study is that we have not been able to confirm our results obtained with the organ bath experiment using more functional and expression studies.
As a result, it is thought that there is no contractile effect of OT over human detrusor muscle. These findings should be supported by further various basic medical or clinical investigations determining the presence of OT receptor in human detrusor tissue.
Disclosure statement
The authors declare that they have no financial disclosures or conflict of interest regarding this manuscript.
References
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683.
- Shojo H, Kaneko Y. Characterization and expression of oxytocin and the oxytocin receptors. Mol Genet Metab. 2000;71(4):552–558.
- Ivell R, Balvers M, Rust W, et al. Oxytocin and male reproductive function. Adv Exp Med Biol. 1997;424:253–264.
- Herbert Z, Botticher G, Aschoff A, et al. Changing caveolin-1 and oxytocin receptor distribution in the ageing human prostate. Anatom Histol Embryol. 2007;36(5):361–365.
- Vignozzi L, Filippi S, Morelli A, et al. Regulation of epididymal contractility during semen emission, the first part of the ejaculatory process: a role for estrogen. J Sex Med. 2008;5(9):2010–2016.
- Bodanszky M, Sharaf H, Roy JB, et al. Contractile activity of vasotocin, oytocin, and vasopressin on mamalian prostate. Eur J Pharm. 1992;216(2):311–313.
- Tarhan F, Ersev D, Aslan N, et al. Oxytocin-induced contractions of rabbit corpus cavernosum. Eur Urol. 1995;28(3):255–258.
- Sanchez M, Andrés-Trelles F, Hidalgo A. Influences of sodium on the contraction induced by oxytocin in rat testicular capsule. Gen Pharmac. 1991;22(4):709–712.
- Romine MT, Anderson GF. Evidence for oxytocin receptors in the urinary bladder of the rabbit. Can J Physiol Pharmacol. 1985;63(4):287–291.
- Pandita RK, Nylen A, Andersson KE. Oxytocin-induced stimulation and inhibition of bladder activity in normal, conscious rats-influence of nitric oxide synthase inhibition. Neuroscience. 1998;85(4):1113–1119.
- Canguven O, Talib R. Are we missing out the role of oxytocin in overactive bladder syndrome? Aging Male. 2019;23:1–5.
- Kimura T, Saji F, Nishimori K. Molecular regulation of the oxytocin receptor in peripheral organs. J Mol Endocrinol. 2003;30:109–115.
- Black LV, Ness TJ, Robbins MT. Effects of oxytocin and prolactin on stress-induced bladder hypersensitivity in female rats. J Pain. 2009;10(10):1065–1072.
- Engle MP, Ness TJ, Robbins MT. Intrathecal oxytocin inhibits visceromotor reflex and spinal neuronal responses to noxious distention of the rat urinary bladder. Reg Anesth Pain Med. 2012;37(5):515–520.