Abstract
Objective
To investigate the time course of total testosterone (TT) recovery after cessation of androgen deprivation therapy (ADT) in Japanese patients treated with brachytherapy.
Methods
In total, 125 patients with prostate cancer received 6 months of neoadjuvant ADT (nADT) followed by low-dose rate (LDR) brachytherapy. TT was measured every 3 months after cessation of nADT, and some predictive factors affecting TT recovery were analyzed.
Results
The cumulative incidence rates of TT recovery to normal levels (TT ≥ 3.0 ng/mL) after 12 and 24 months cessation were 49.6% and 81.6%, respectively. The median interval to recover to normal TT was 15 months. In multivariate analysis, the use of a gonadotropin-releasing hormone (GnRH) antagonist as nADT significantly earlier improved to recovery to normal TT level (p = 0.046). Conversely, higher body mass index (BMI) and hypertension significantly prolonged TT recovery to normal (p = 0.026 and p = 0.026, respectively).
Conclusions
Approximately one-fifth of patients still had low TT levels 2 years after the cessation of 6 months nADT before LDR brachytherapy. Use of a GnRH agonist, higher BMI, and hypertension were the predictive factors for slower TT recovery to normal TT levels after the cessation of nADT.
Introduction
Androgen deprivation therapy (ADT) is widely operated as a neoadjuvant therapy with various radiotherapies for the management of locally advanced or localized unfavorable-risk prostate cancer [Citation1]. The planned purpose and duration of ADT depends on the local extent of the cancer and the type of radiation therapy [Citation2]. According to some randomized controlled studies, the use of 6–36 months of ADT with external beam radiotherapy (EBRT), as compared with the use of radiotherapy alone, contributed to a significant improvement in overall survival and cancer-specific survival among men with locally advanced or localized unfavorable-risk prostate cancer [Citation3].
After cessation of ADT, serum total testosterone (TT) levels usually recover from castrated levels to normal levels. However, some patients maintain low TT levels for several months after cessation. In these cases, patients remain affected by hypogonadism syndrome, despite the discontinuance of ADT. ADT adversely affects hypogonadal symptoms such as erectile function, sexual desire, arousal ability, menopausal symptoms, and metabolic syndrome [Citation4]. Low testosterone after the cessation of neoadjuvant ADT (nADT) for radiotherapy is continued for longer periods, potentially affecting hypogonadism syndrome [Citation5]. Therefore, neoadjuvant regimens that have fewer effects on testosterone recovery should be recommended to maintain quality of life (QOL) in prostate cancer patients.
The course of testosterone recovery after the cessation of neoadjuvant gonadotropin-releasing hormone (GnRH) agonist or antagonist treatment before radiotherapy has been previously reported [Citation6]. However, information regarding predictive factors affecting testosterone recovery after the cessation of nADT before brachytherapy has been lacking, and it is likely to be clinically useful to identify predictive factors associated with testosterone recovery.
The present study investigated the time course of recovery of normal TT levels after cessation of 6 months nADT in patients treated with low-dose rate (LDR) brachytherapy for prostate cancer. In addition, we aimed to determine predictive factors that affect the time course of recovery to normal TT levels after the cessation of nADT.
Materials and methods
Study patients
In total, 125 patients who received 125I permanent LDR brachytherapy for prostate cancer with 6 months nADT using GnRH agonist or antagonists between October 2007 and September 2016 at our institution were enrolled. The TT level of each patient was measured at the cessation of nADT and until it recovered to a normal level. A follow-up examination after the cessation of nADT was scheduled every 3 months. A normal level of TT was defined as over 3.0 ng/mL as the 2018 American Urological Association (AUA) guideline on the evaluation and management of testosterone deficiency which recommends that 3.0 ng/mL is used as the threshold for prescribing testosterone replacement therapy (TRT) [Citation7]. A castrated level of TT level was defined as under 0.5 ng/mL [Citation8]. Serum TT levels were measured by enzyme immunoassay. The study was conducted in accordance with the Declaration of Helsinki and approved by the Kanazawa University Hospital ethics committee (approval no. 2018-275).
Study protocol
All patients underwent a complete history and physical examination at the beginning of nADT, including body mass index (BMI) and the presence of obesity, hypertension, and diabetes. Obesity was defined as BMI ≥ 25 according to the Japanese criteria proposed by the Examination Committee of Criteria for the diagnosis of obesity in Japan [Citation9]. Hypertension and diabetes were diagnosed as follows: hypertension (systolic blood pressure [SBP] ≥ 140 mmHg or diastolic blood pressure [DBP] ≥ 90 mmHg or the use of antihypertensive medication) [Citation10] and diabetes (fasting plasma glucose [FPG] ≥ 126 mg/dl or hemoglobin A1c [HbA1c] ≥ 6.5%) [Citation11]. In all cases, nADT was the combined androgen blockade therapy, consisted of either a GnRH agonist as a 1 or 3 months formulation with an oral anti-androgen or a GnRH antagonist as a 1 months preparation with an oral anti-androgen. In all cases, bicalutamide (80 mg) was used as the oral nonsteroidal anti-androgen agent. Goserelin (3.6 or 10.8 mg) or leuprolide (3.75 or 11.25 mg) was administrated as the GnRH agonist, and degarelix (240 mg at first dose, 80 mg thereafter) was administered as the GnRH antagonist. All patients were candidates for LDR brachytherapy combined with 6 months nADT. The prescribed dose to the periphery of the prostate was 110 Gy using a prostate implant technique reported previously [Citation12,Citation13]. In addition, patients with high-risk prostate cancer according to the D’Amico criteria were administered EBRT subsequently to LDR brachytherapy [Citation13]. Patients received EBRT using a dynamic-arc conformal technique and were administered high-energy photons comprising 10 MV X-rays. The radiation field was limited to the prostate gland.
Statistical analysis
The Kaplan–Meier method was used to estimate the cumulative incidence of TT recovery. In univariate analysis, the Mann–Whitney U test and Fisher exact probability test were used to determine the factors affecting TT recovery. Multivariate logistic regression models were created on the basis of the covariates that were significant in the univariate analysis. All statistical analyses were performed using SPSS™ statistics 22 (SPSS Inc., Chicago, IL). In all analyses, p-values < 0.05 were considered statistically significant.
Results
The median age (range) at entry was 68 years (55–79), and the median BMI at the beginning was 24.1 (16.6–34.0) (). The median serum PSA (prostate-specific antigen) level at diagnosis was 7.7 ng/mL (2.03–85.0). The number of subjects who underwent EBRT following LDR was 32, and the number of high-risk prostate cancer patients was 35. In total, 66 patients were administered a GnRH agonist as nADT, and 59 were administrated a GnRH antagonist. At the cessation of nADT, it was confirmed that all patients reached castrated TT levels (TT < 0.5 ng/mL).
Table 1. Patient backgrounds.
We estimated the cumulative incidence of TT recovery to normal levels at 12 and 24 months after cessation as 49.6 and 81.6%, respectively (). The median interval to recover the normal TT level was 15 months.
Figure 1. Cumulative incidence of TT recovery to normal levels (≥3.0 ng/mL). TT: total testosterone.
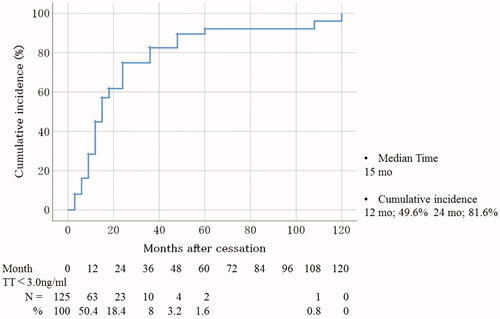
To identify the predictive factors of TT recovery, patients were divided into two groups according to the median of the interval to TT normalization after cessation of ADT. Patients whose TT recovered to normal levels within 15 months of cessation were assigned to the TT recover group (n = 57; 47.5%), whereas the subjects whose TT remained below normal levels after 15 months were attached to the not TT recover group (n = 63; 52.5%) (). We were unable to assess whether TT levels improved within 15 months in five patients because of lacking data. BMI was significantly lower in the TT recover group than in the not TT recover group (p = 0.046). However, the number of subjects with obesity (BMI ≥ 25) did not differ significantly between the two groups. The number of patients who were administered a GnRH antagonist was significantly more in the TT recover group compared to that in the not TT recover group (p = 0.0056). Furthermore, the number of patients with EBRT followed by brachytherapy, high-risk prostate cancer, and current history of hypertension was significantly higher in the not TT recover group than in the TT recover group (p = 0.013, p = 0.0025, and p = 0.0097, respectively). No statistically significant differences were observed between the two groups in any other characteristic.
Table 2. Comparisons of background data of patients divided into two groups according to the interval to TT normalization after cessation (15 months).
On multivariate analysis, higher BMI and hypertension were significantly associated with slower recovery to normal TT levels [odds ratio (OR) = 0.846; 95%CI = 0.731–0.980; p = 0.026 and OR = 0.388; 95%CI = 0.168–0.894; p = 0.026, respectively] (). In addition, the usage of a GnRH antagonist as nADT significantly influenced more rapid recovery to normal TT levels (OR = 2.284; 95%CI = 1.014–5.144; p = 0.046).
Table 3. Multivariate analyses of predictive factors associated with TT normalization.
Discussion
In the present study, we estimated the time course of recovery of TT levels after cessation of 6 months use of nADT in prostate cancer patients treated with LDR brachytherapy. Approximately 20% of the patients did not experience serum TT normalization at 2 years after cessation. We must make every effort to limit the adverse effects associated with hypogonadism caused by these treatments for prostate cancers. The present study demonstrated that the use of a GnRH agonist, higher BMI, and hypertension was significantly associated with earlier serum TT normalization after ADT cessation.
Several reports have previously described the recovery rate of TT level after ADT [Citation14]. Inoue et al. [Citation15] prospectively investigated the timing and extent of TT recovery in 10 patients with intermediate-risk prostate cancer who received EBRT combined with 4 months GnRH antagonist administration. In their cohort, the median time to testosterone normalization was 7 months, and 80% of their testosterone levels had normalized within 9 months after the last administration of the GnRH antagonist. This earlier improved TT recovery compared with that in the present study was almost certainly due to differences in the definition of normal TT levels (1.29 vs. 3.0 ng/mL). The 2018 AUA guideline on the evaluation and management of testosterone deficiency recommends that a 300 ng/dL TT level should be used as the threshold for prescribing TRT [Citation7]. In other words, we have to take care of patients whose serum TT is under 300 ng/dL in clinical settings. Inoue et al. [Citation15] also reported that the deterioration of testicular function persisted 1 year after the last GnRH antagonist administration and that the sexual and hormonal function estimated using patient-reported outcome measures (PROMs) declined after GnRH antagonist treatment but gradually recovered within 15 months of the last administration [Citation15], suggesting that the symptoms or conditions associated with TT deficiency after neoadjuvant GnRH antagonist administration, persisted from 12 to 15 months; this was similar to the time to TT normalization in our cohort.
Prolonged low TT levels could maintain long-term negative effects for patients with ADT and may result in increased development of cardiovascular events, diabetes, and osteoporotic fracture [Citation16]. The osteoporotic fractures caused by ADT can potentially affect QOL and disease prognosis in patients with prostate cancer, and the mortality rate was 2-fold greater in men who experienced a fracture after diagnosis compared with that in men who did not [Citation17]. Daisuke Watanabe et al. [Citation18] have investigated the influence of ADT on the hip geometric properties evaluated by dual-energy X-ray. In their study, a total of 65 Japanese men with prostate cancer who underwent ADT for the first time were included. The hip geometric parameters and the bone mineral density (BMD) taken before and after 1 year of ADT were retrospectively examined. With ADT, they not only confirmed significant BMD annual changes in the lumbar spine, the femoral neck, and the total hip of −1.65%, −1.91%, and −2.20%, respectively, but they also confirmed significant annual changes in cross-sectional areas, cross-sectional moments of inertia, and section modulus in the narrow femoral neck of −2.55%, −3.50%, and −3.14%, respectively [Citation18]. Furthermore, a lower TT level is associated with cardiovascular events in middle-aged Japanese men, independent of coronary risk factors and endothelial dysfunction [Citation19]. The subjects with the lowest tertial of plasma TT (<4.10 ng/mL) had a significantly higher (4-fold) risk for cardiovascular event compared with those with the higher testosterone tertial (>5.60 ng/mL) after adjustment for some coronary risk factors including medications and flow-mediated dilation of the brachial artery [Citation19]. Liao et al. [Citation20] also have stated the association between serum testosterone levels and acute ischemic stroke in males. According to their cohort, bioavailable and free testosterone are significantly associated with the 3 months the modified Rankin Scale after acute ischemic stroke in male patients only in univariate analysis but not in multivariate analysis [Citation20]. Therefore, the management of TT recovery is important in patients who undergo ADT, and clinicians should consider regimens of nADT to shorten the period of low TT after ADT cessation wherever possible.
Tsumura et al. [Citation21] previously reported an investigation of TT recovery after the cessation of nADT in Japanese prostate cancer patients who had undergone brachytherapy. The median time to testosterone normalization (defined as ≥2.07 ng/mL) in their cohort [Citation21] was the same as that in Inoue et al’s cohort [Citation15] according to the Kaplan–Meier curve of the cumulative incidence of TT recovery. They also analyzed the predictive factors affecting TT recovery, including BMI, age and TT level at ADT cessation; however, no predictive factors associated with TT recovery could be determined [Citation21]. On the other hand, in addition to the use of a GnRH agonist, we could identify higher BMI and hypertension as predictive factors that contributed to slower TT normalization.
In contrast to our cohort, age has been reported as a predictor associated with TT recovery in other studies [Citation22,Citation23]. The subject’s BMI, the proportion of the use of GnRH agonist or antagonist and the ratio of hypertension varied between ages in this study. Therefore, age may not be a significant factor for TT normalization because the use of a GnRH agonist, hypertension and BMI may strongly affect TT recovery.
Higher BMI has been widely accepted as an important factor for testosterone deficiency [Citation24]; conversely, a low TT level could be also associated with obesity [Citation25]. A previous study has demonstrated that the elevation of serum leptin associated with obesity inhibits the function of Leydig cells, resulting in a decrease in gonadotropin-stimulated androgen production [Citation26]. Furthermore, adipocytes have high expression of aromatase that enzymatically converts testosterone to estradiol and thus lowers circulating androgens. Concurrently, estrogens act on the hypothalamic-pituitary axis as a negative feedback mechanism to suppress GnRH and subsequent luteinizing hormone (LH), leading to a reduction in gonadal testosterone release [Citation27]. Therefore, obesity can directly impact on testosterone levels. In Japan, obesity is more severe than WHO standards (defined as BMI ≥ 25 in Japan vs. 30 in WHO) [Citation9,Citation28]. In fact, only 4.9% of patients (n = 6; 6/125) was had BMI ≥ 30 in this study. Although the present study demonstrated that higher BMI is certainly an important factor for delay of TT recovery, the number of the subjects with obesity (BMI ≥ 25) did not differ significantly between the two groups because of a limited number of patients with severe obesity.
The present study found hypertension as another predictive factor for slower TT recovery. The individual association of low TT level with hypertension has been also demonstrated previously [Citation29]. Testosterone induces antihypertensive action according to these previous studies, and it has been widely documented that testosterone can regulate the cardiovascular system through both genomic (anti-inflammatory activity) and nongenomic (vasorelaxing effect in isolated blood vessels from several species) mechanisms [Citation30]. However, these findings cannot directly explain the reasons why hypertension had a negative effect on TT recovery, and further studies are necessary to clarify this mechanism.
GnRH antagonists induced TT and PSA suppression significantly faster than did GnRH agonists [Citation31]. GnRH agonists are initially associated with stimulation of the pituitary release of LH, causing an increase in testicular androgen production. Continued administration results in desensitization of pituitary GnRH receptors by down-regulation, contributing to a decline in LH release and suppression of gonadal androgen synthesis [Citation32]. On the other hand, GnRH antagonists rapidly decrease testicular testosterone secretion from the Leydig cells of the testicles by blocking the interaction of LH from the pituitary [Citation33]. We suppose that the difference in the mechanisms involved in reducing TT between GnRH antagonists and agonists is likely to affect TT recovery after the cessation of ADT. Alternatively, the different bioavailability of GnRH antagonists and agonists could also explain the difference in the recovery of TT. Degarelix used as a GnRH antagonist must be given every 4 weeks, whereas leuprolide and goserelin administered as GnRH agonists can be given every 1–6 months. Differences in half-life between pharmaceutical formulations may also impact TT recovery.
The present study has some limitations. Previous research suggested the impact of scatter radiation on TT levels and Leydig cell function in prostate cancer patients with EBRT [Citation34]. However, it is still unclear how brachytherapy itself can affect TT levels. Thus, the cumulative incidence of TT recovery might be incommensurable among patients undergoing nADT and brachytherapy. We measured serum TT levels by using enzyme immunoassay approved by the Japanese health insurance system. The coefficients of variation are around 25% according to the previous studies [Citation35,Citation36], which may be the bias of the present research. Furthermore, we could not evaluate the influence of baseline TT levels before nADT on TT recovery because of the lack of data. Patients with baseline TT < 3.0 ng/mL before treatment could not understandably achieve TT recovery to normal range after ADT. On the other hand, according to the previous epidemiological study, baseline TT hardly ever decreases with age in the Japanese population [Citation37]. This previous study has reported the TT remained almost unchanged in subjects aged 50 and above among 1143 Japanese men; mean TT values ± SD were 3.91 ± 0.92 in the 50s, 3.83 ± 0.90 in 60s, and 3.84 ± 0.95 in 70s. In the present cohort, all subjects were over 50 years. Therefore, we sufficiently expect that not many patients had a baseline TT ≤ 3.0 ng/mL at any age. In addition, we did not investigate the impact of the prolonged low TT condition after nADT on the QOL among patients with prostate cancer. Given that this was a retrospective study, further prospective studies including patients’ background data and QOL assessments are required to reach a more definite conclusion.
Approximately one-fifth of patients still had low TT levels 2 years after the cessation of 6 months nADT before LDR brachytherapy. Use of a GnRH agonist, higher BMI, and hypertension were the predictive factors for slower TT recovery to normal TT levels after the cessation of nADT.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451–459.
- Monroe AT, Faricy PO, Jennings SB, et al. High-dose-rate brachytherapy for large prostate volumes (≥50cc)—uncompromised dosimetric coverage and acceptable toxicity. Brachytherapy. 2008;7(1):7–11.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106.
- Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836.
- Donovan KA, Walker LM, Wassersug RJ, et al. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer. 2015;121(24):4286–4299.
- Shahidi M, Norman AR, Gadd J, et al. Recovery of serum testosterone, LH and FSH levels following neoadjuvant hormone cytoreduction and radical radiotherapy in localized prostate cancer. Clin Oncol. 2001;13(4):291–295.
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200(2):423–432.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642.
- The Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66(11):987–992.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81–S90.
- da Silva Franca CA, Vieira SL, Carvalho AC, et al. Localized prostate cancer with intermediate- or high-risk features treated with combined external beam radiotherapy and iodine-125 seed brachytherapy. Brachytherapy. 2010;9(4):307–312.
- Zilli T, Boudreau C, Filion EJ, et al. Combined intensity-modulated radiation therapy vs. three-dimensional highly conformal radiotherapy after 125I prostate permanent seed brachytherapy: a comparative treatment planning study. Brachytherapy. 2011;10(5):416–420.
- Padula GDA, Zelefsky MJ, Venkatraman ES, et al. Normalization of serum testosterone levels in patients treated with neoadjuvant hormonal therapy and three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52(2):439–443.
- Inoue T, Mizowaki T, Kabata D, et al. Recovery of serum testosterone levels and sexual function in patients treated with short-term luteinizing hormone-releasing hormone antagonist as a neoadjuvant therapy before external radiotherapy for intermediate-risk prostate cancer: preliminary prospective study. Clin Genitourin Cancer. 2018;16(2):135.e1–141.e1.
- Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456.
- Beebe-Dimmer JL, Cetin K, Shahinian V, et al. Timing of androgen deprivation therapy use and fracture risk among elderly men with prostate cancer in the United States. Pharmacoepidemiol Drug Saf. 2012;21(1):70–78.
- Watanabe D, Kimura T, Yamashita A, et al. The influence of androgen deprivation therapy on hip geometric properties and bone mineral density in Japanese men with prostate cancer and its relationship with the visceral fat accumulation. Aging Male. [cited 2020 Jan 20]; [7 p.]. DOI:10.1080/13685538.2020.1713741
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210(1):232–236.
- Liao PW, Chen JT, Liu SP, et al. The predictive value of serum testosterone level on the functional outcomes after acute ischemic stroke in males. Aging Male. [cited 2019 Mar 29]; [7 p.]. DOI:10.1080/13685538.2019.1582620
- Tsumura H, Satoh T, Ishiyama H, et al. Recovery of serum testosterone following neoadjuvant and adjuvant androgen deprivation therapy in men treated with prostate brachytherapy. World J Radiol. 2015;7(12):494–500.
- Nascimento B, Miranda EP, Jenkins LC, et al. Testosterone recovery profiles after cessation of androgen deprivation therapy for prostate cancer. J Sex Med. 2019;16(6):872–879.
- Kaku H, Saika T, Tsushima T, et al. Time course of serum testosterone and luteinizing hormone levels after cessation of long-term luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Prostate. 2006;66(4):439–444.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91(11):4335–4343.
- Corona G, Mannucci E, Fisher AD, et al. Low levels of androgens in men with erectile dysfunction and obesity. J Sex Med. 2008;5(10):2454–2463.
- Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84(10):3673–3680.
- Cohen PG. The hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt–a major factor in the genesis of morbid obesity. Med Hypotheses. 1999;52(1):49–51.
- World Health Organization. Obesity: preventing and managing the global epidemic. Geneva (Switzerland): Division of Noncommunicable Disease, Programme of Nutrition Family and Reproductive Health, World Health Organization; 1998. (Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997).
- Yang Q, Li Z, Li W, et al. Association of total testosterone, free testosterone, bioavailable testosterone, sex hormone-binding globulin, and hypertension. Medicine. 2019;98(20):e15628.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318.
- Clinton TN, Woldu SL, Raj GV. Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer. Expert Opin Pharmacother. 2017;18(8):825–832.
- Chrisp P, Sorkin EM. Leuprorelin. A review of its pharmacology and therapeutic use in prostatic disorders. Drugs Aging. 1991;1(6):487–509.
- Broqua P, Riviere PJ, Conn PM, et al. Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther. 2002;301(1):95–102.
- Izard MA. Leydig cell function and radiation: a review of the literature. Radiother Oncol. 1995;34(1):1–8.
- Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography–mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49(8):1381–1395.
- La’ulu SL, Kalp KJ, Straseski JA. How low can you go? Analytical performance of five automated testosterone immunoassays. Clin Biochem. 2018;58:64–71.
- Iwamoto T, Yanase T, Koh E, et al. Reference ranges of total serum and free testosterone in Japanese male adults. Jap J Urol. 2004;95(6):751–760.
