Abstract
Aim
To investigate if overweight and obesity were associated with a higher degree of biochemical recurrence (BCR) after radical prostatectomy, in Mexican men with prostate cancer (PCa).
Methods
We included 180 men with PCa, who underwent radical prostatectomy (RP). Body mass index (BMI) was determined and the degree of PCa aggressiveness was established according to the D’Amico classification. Postoperative follow-up of all patients was performed with PSA quantification every/6 weeks after surgery and then at 3-month intervals for 1 year, followed every/6 months for 5 years. Postoperative BCR was defined as two consecutive increases in PSA levels ≥0.4 ng/mL, after RP.
Results
Sixty eight percent of the patients presented overweight or obesity. We found that only intermediate/high risk patients presented an increased risk factor for BCR-free survival (HR = 4.39; 95% CI = 1.74–11.24; p = 0.002). The median follow-up of all men has been 7.9 years and no significant differences in BCR-free survival time has been observed between the BMI groups.
Conclusions
The overweight and obesity do not represent a risk factor to present BCR after RP for PCa. However, an intermediate/high risk, according to the D’Amico’s classification, constitutes a risk factor to present BCR after radical prostatectomy, which is not related to the BMI.
Introduction
Overweight and obesity have reached epidemic proportions. In México, the last National Health and Nutrition Survey demonstrated that overweight and obesity prevalence in men is 42.5% and 30.5%, respectively [Citation1]. It has been suggested that obesity may play a role in the progression of PCa, rather than increasing its onset [Citation2]. Several studies have indicated that obesity could be associated with an increased risk of developing an aggressive form of prostate cancer, consequently increasing its mortality rate [Citation3–5]. Besides, it has been proposed that obesity increases the risk of biochemical recurrence rates ) (BCR), tumor aggressiveness, and a higher prostate cancer-specific mortality [Citation5–11].
On the other hand, prostate cancer (PCa) represents the most common solid malignancy among men worldwide, with an estimated 1.3 million cases diagnosed each year. In Mexico, it represents the first cause of cancer deaths in men, with a mortality rate, during 2018, of 10 deaths per 100,000 men and 25,049 new cases per year [Citation12].
Zhang et al. [Citation13] have indicated that detection of NF-kappaB-mediated Transforming growth factor-beta (TGF-beta)-mediated epithelial-to-mesenchymal transition (EMT), in primary tumors, predicts disease recurrence in prostate cancer patients after radical prostatectomy. Changes in TGF-beta signaling and EMT-related factors, provide novel molecular markers which could be used as prognosis factors after treatment. Moreover, Torrealba et al. [Citation14], analyzed the relationship of components of transforming growth factor-B (TGF-b)/phosphoinositide-3-kinases (PI3K)/AKT/mammalian target of rapamycin (mTOR)/nuclear factor kappa B (NF-kB) transduction pathway in patients with prostate cancer and their relation with clinicopathologic characteristics. These authors suggested that TGFBR1 and PI3K could be prognostic and independent markers of biochemical progression in patients with this cancer. However, mechanisms that relate increased body adiposity with PCa are complex and have not been completely elucidated; however, dysregulation in the insulin/IGF-1 axis [Citation15–17] and in adipokine signaling [Citation18,Citation19], have been implicated in the severity of this cancer.
Since obesity has been considered as an independent risk factor for the development of aggressive forms of prostate cancer, we investigated if overweight and obesity were associated with biochemical recurrence after radical prostatectomy, in Mexican men with prostate cancer.
Subjects and methods
Subjects
The study was approved by the Human Research Committees of the participating institutions and informed written consent was obtained from all men before participation. One hundred eighty unrelated men, with histologically confirmed PCa, who underwent radical prostatectomy (RP), were recruited. Clinical and pathological characteristics, as well as PCa outcomes were reviewed from medical records. Men with a history of other previous neoplasias or with a family history of prostate cancer, as well as patients with incomplete clinical data or with a follow-up less than 5 years were excluded.
Body mass index (BMI) (kg/m2) was calculated and patients were categorized as having a normal BMI if it was in the range of 18.5–24.9 kg/m2, being overweight if BMI was in the range of 25.0–29.9 kg/m2 and being obese if BMI was ≥30.0 kg/m2 [Citation20].
Besides, patients were categorized according to the classification proposed by D’Amico into low, intermediate and high risk, based on their prostate-specific antigen (PSA) concentrations, Gleason grade and clinical stage [Citation21].
Postoperative follow-up of all patients was performed with PSA quantification every 6 weeks after surgery and then at 3-month intervals for 1 year, followed every 6 months for 5 years. Postoperative BCR was defined as two consecutive increases in PSA levels ≥0.4 ng/mL, after RP.
Statistical analysis
Data were summarized as median and range, in the case of quantitative variables and absolute and relative frequencies for qualitative variables. Data distribution was tested using the Kolmogorov-Smirnov test. Continuous and categorical variables were compared among BMI groups, using one-way analysis of variance (ANOVA) or a Kruskal Wallis test (for continuous variables) and a χ2 or Fisher exact test (for categorical variables).
Kaplan-Meier with log-rank test analyses were performed to test the effect of each BMI group on BCR-free survival; multivariate analyses were conducted using Cox proportional-hazards regression models in order to recognize potential confounder variables. Furthermore, as Catalona et al. [Citation22] demonstrated that patients older than 70 years exhibit a more advanced cancer, we analyzed the effect that age could have (cutoff point 70 years old) on BCR-free survival, regarding different BMI.
Statistical analyses were carried out using STATA 13.0© (Texas, USA); a p value <0.05 was accepted as statistically significant.
Results
One hundred eighty men (45–79 years old) presenting different BMI and a localized prostate adenocarcinoma, treated with RP, were included. According to the World Health Organization criteria, 57 (31.7%) had a normal BMI, and 123 (68.3%) presented overweight or obesity. General characteristics of all men with PCa, grouped according to their BMI, are shown in .
Table 1. Clinicopathologic characteristics of Mexican men with prostate cancer and different body mass indexes.
Regarding cancer aggressiveness, according to the D’Amico’s classification, the intermediate/high risk was similar in both groups (68.3% in overweight and obesity patients, vs 66.7% in normal weight patients) (). Besides, we observed that after prostatectomy, patients with overweight or with obesity had similar concentrations of PSA, when compared to patients with a normal BMI (). We also found that men with overweight or with obesity harbored extra-prostatic invasion more frequently, as well as lymph node metastases, when compared with men with normal BMI; however, this difference was not statistically significant ().
Cox proportional hazards analysis was performed to test the association of different risk factors (including overweight and obesity) with BCR-free survival. We found that only intermediate/high risk classified patients (D’Amico’s) presented an increased risk factor for BCR-free survival (HR = 4.39; 95% CI = 1.74–11.24; p = 0.002) ().
Table 2. Cox proportional hazards analysis for biochemical recurrence free survival after radical prostatectomy in Mexican men with prostate cancer.
To date, the median follow-up of all men has been 7.9 years, and biochemical recurrence has been recorded in 24 (42.1%) men with normal BMI and in 51 (41.5%) patients with overweight or with obesity. No significant differences in BCR-free survival time was observed between the BMI groups ().
Figure 1. Kaplan-Meier survival estimates for biochemical recurrence free survival by body mass index (BMI). No association was found between overweight/obesity and biochemical recurrence free survival decreases.
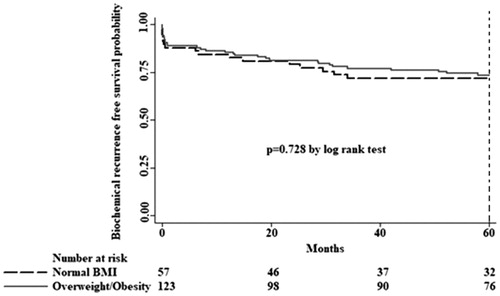
Moreover, we stratified patients with PCa into two subgroups of age: ≤70 years (N = 150) and older than 70 years (N = 30) and we also stratified them taking into account their different body mass indexes. Those men older than 70, presented a normal BMI in only 8 cases, whilst 22 had overweight or obesity. After the analysis with Cox proportional hazards, for risk factors (BMI, D’Amico classification, extra-prostatic invasion, lymph node metastases or positive surgical margins), we did not find any association with BCR-free survival (data not shown).
Discussion
Since obesity and overweight have reached epidemic proportions, being México the second nation with the biggest obesity prevalence worldwide [Citation1] and because PCa represents the first cause of cancer deaths in Mexican men [Citation12], we investigated if overweight and obesity are associated with aggressiveness and biochemical recurrence, after radical prostatectomy in patients with prostate cancer.
Schiffmann et al. [Citation23], examined the effect of obesity on PCa aggressiveness, observing that patients with obesity harbored a more aggressive cancer at diagnosis, characterized by more lymph node metastases and a positive surgical margin, when compared to men with normal BMI. Interestingly, we found that men with overweight or with obesity harbored extra-prostatic invasion more frequently, as well as lymph node metastases, when compared with men with normal BMI; however, this difference was not statistically significant.
The relationship between obesity and BCR after radical prostatectomy for PCa cancer is controversial. Tomaszewski et al. [Citation24], performed a 12-year retrospective study, in which they analyzed if individuals with obesity presented aggressive forms of PCa. The authors found no association between overweight or obesity with adverse pathologic characteristics, positive surgical margins, greater biochemical recurrence rates or interval to death after primary treatment with radical prostatectomy. On the other hand, Hu et al. [Citation6] carried out a meta-analysis in which they evaluated 36,927 individuals with PCa, demonstrating that a 5 kg/m2 increase in BMI was associated with a higher risk (16%) of BCR. Moreover, Schiffmann et al. [Citation23] examined the effect of obesity on BCR rates, finding that there were no significant differences in BCR-free survival between obese and nonobese men. These authors concluded that although patients with obesity harbored more aggressive PCa at diagnosis, obesity was not an independent predictor of BCR after RP. Likewise, Schiffmann et al. [Citation25] investigated the association of obesity on PCa progression after treatment with RP, indicating that patients with obesity presented a lower risk of metastases; as well as, increased metastases-free survival in comparison to patients with normal BMI after treatment with RP. Regarding our results, we also did not find that individuals with overweight and obesity presented metastases or greater biochemical recurrence rates after RP.
Like it has been proposed by Schiffmann et al. [Citation23], our results could be due to the fact that once treatment with RP has been performed, the increased risk related to obesity might be neutralized. This implies that once treatment is administered, the increased risk related to overweight and/or obesity disappears.
In addition, our data might indicate an obesity paradox as has been previously suggested in other types of cancer [Citation26–28], as well as in PCa, since it has been reported that patients with overweight or with obesity have a lower risk of metastasis after RP, in comparison to men with normal BMI [Citation25]. This obesity paradox has been described in cardiovascular and metabolic research [Citation29–31].
On the other hand, it has been described that individuals with advanced age present more aggressive forms of PCa after RP [Citation32–35]; being the age group of 70–74, the one with the highest incidence [Citation36]. This fact is important, since data provided by the National Institute of Statistics and Geography (INEGI) (https://www.inegi.org.mx/temas/estructura/) demonstrates that in Mexico men older than 70 years, represent 4.5% of our population. Furthermore, overweight and obesity prevalence in men older than 70 years in Mexico is 41.3% and 14.9%, respectively [Citation37].
Brassell et al. [Citation33], conducted a study in which they evaluated the clinicopathologic characteristics of PCa in men 70 years of age or older. After prostatectomy these authors observed that this group of patients presented a higher BCR. Likewise, Ko et al. [Citation34] found that men older than 70 years and treated with RP, presented a more aggressive PCa when compared to men 50–70 years old, with similar PCa pathologic characteristics at RP. However, we did not find an association among aggressiveness or BCR-free survival of PCa, in men >70 years with overweight and obesity; however, it is noteworthy that our population of men over 70 years was very small.
This study had limitations. One is that it is a retrospective study. Another limitation was that we did not have the power to detect the effect of overweight and obesity on the BCR-free survival or aggressiveness of the PCa. Despite these limitations, the strengths of the study were that all patients had more than 5 years of follow up.
In conclusion, we found that overweight and obesity do not represent a risk factor to present biochemical recurrence after radical prostatectomy for PCa. However, the presence of an intermediate/high risk according to the D’Amico’s classification constitutes a risk factor to present biochemical recurrence after radical prostatectomy. We consider that further studies should be directed to determine if overweight and/or obesity, in men older than 70 years, constitute a risk factor for biochemical recurrence of prostate cancer.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals included in this study.
Disclosure statement
All authors declare that there are no conflicts of interest.
References
- Encuesta Nacional de Salud y Nutrición (ENSANUT). México: Secretaría de Salud, Instituto Nacional De Salud Pública, Instituto Nacional de Estadística y Geografía, México 2018. https://ensanut.insp.mx/
- Golabek T, Bukowczan J, Chlosta P, et al. Obesity and prostate cancer incidence and mortality: a systematic review of prospective cohort studies. Urol Int. 2014;92(1):7–14.
- Cantarutti A, Bonn SE, Adami HO, et al. Body mass index and mortality in men with prostate cancer. Prostate. 2015;75(11):1129–1136.
- Hu MB, Xu H, Zhu WH, et al. High-fat diet-induced adipokine and cytokine alterations promote the progression of prostate cancer in vivo and in vitro. Oncol Lett. 2017;15(2):1607–1615.
- Dickerman BA, Torfadottir JE, Valdimarsdottir UA, et al. Body fat distribution on computed tomography imaging and prostate cancer risk and mortality in the AGES-Reykjavik study. Cancer. 2019;125(16):2877–2885.
- Hu MB, Xu H, Bai PD, et al. Obesity has multifaceted impact on biochemical recurrence of prostate cancer: a dose-response meta-analysis of 36,927 patients. Med Oncol. 2014;31(2):829.
- Vidal AC, Howard LE, Moreira DM, et al. Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2936–2942.
- Yamoah K, Zeigler-Johnson CM, Jeffers A, et al. The impact of body mass index on treatment outcomes for patients with low-intermediate risk prostate cancer. BMC Cancer. 2016;16(1):557.
- Vidal AC, Howard LE, Sun SX, et al. Obesity and prostate cancer-specific mortality after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Prostate Cancer Prostatic Dis. 2017;20(1):72–78.
- Freedland SJ, Branche BL, Howard LE, et al. Obesity, risk of biochemical recurrence, and prostate-specific antigen doubling time after radical prostatectomy: results from the SEARCH database. BJU Int. 2019;124(1):69–75.
- Wissing M, Chevalier S, McKercher G, et al. The relationship between body-mass index, physical activity, and pathologic and clinical outcomes after radical prostatectomy for prostate cancer. World J Urol. 2019;37(5):789–798.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Zhang Q, Helfand BT, Jang TL, et al. Nuclear factor-κB-mediated transforming growth factor-β-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2009;15(10):3557–3567.
- Torrealba N, Vera R, Fraile B, et al. TGF-β/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer. Aging Male. 2019;1–11. DOI:10.1080/13685528.2019.1597840
- DiGiovanni J, Kiguchi K, Frijhoff A, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97(7):3455–3460.
- Roddam AW, Allen NE, Appleby P, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149(7):461–471, w83–w88.
- Rabin-Court A, Rodrigues MR, Zhang XM, et al. Obesity-associated, but not obesity-independent, tumors respond to insulin by increasing mitochondrial glucose oxidation. PLOS One. 2019;14(6):e0218126.
- Gao Q, Zheng J, Yao X, et al. Adiponectin inhibits VEGF-A in prostate cancer cells. Tumor Biol. 2015;36(6):4287–4292.
- Tan W, Wang L, Ma Q, et al. Adiponectin as a potential tumor suppressor inhibiting epithelial-to-mesenchymal transition but frequently silenced in prostate cancer by promoter methylation. Prostate. 2015;75(11):1197–1205.
- World Health Organization 2018. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
- Catalona WJ, Hudson MA, Scardino PT, et al. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152(6 Part 1):2037–2042.
- Schiffmann J, Salomon G, Tilki D, et al. Radical prostatectomy neutralizes obesity-driven risk of prostate cancer progression. Urol Oncol. 2017;35(5):243–249.
- Tomaszewski JJ, Chen YF, Bertolet M, et al. Obesity is not associated with aggressive pathologic features or biochemical recurrence after radical prostatectomy. Urology. 2013;81(5):992–996.
- Schiffmann J, Karakiewicz PI, Rink M, et al. Obesity paradox in prostate cancer: increased body mass index was associated with decreased risk of metastases after surgery in 13,667 patients. World J Urol. 2018;36(7):1067–1072.
- Lennon H, Sperrin M, Badrick E, et al. The Obesity Paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56.
- Renehan AG, Sperrin M. The Obesity Paradox and mortality after colorectal cancer: a causal conundrum. JAMA Oncol. 2016;2(9):1127–1129.
- Ujvari B, Jacqueline C, Misse D, et al. Obesity paradox in cancer: is bigger really better? Evol Appl. 2019;12(6):1092–1095.
- Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364(8):719–729.
- Lainscak M, von Haehling S, Doehner W, et al. The obesity paradox in chronic disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2012;3(1):1–4.
- Doehner W, von Haehling S, Anker SD. Protective overweight in cardiovascular disease: moving from ‘paradox’ to ‘paradigm’. Eur Heart J. 2015;36(40):2729–2732.
- Huang SP, Huang CY, Liu CC, et al. Clinical outcome of Taiwanese men with clinically localized prostate cancer post-radical prostatectomy: a comparison with other ethnic groups. Aging Male. 2010;13(1):10–17.
- Brassell SA, Rice KR, Parker PM, et al. Prostate cancer in men 70 years old or older, indolent or aggressive: clinico pathological analysis and outcomes. J Urol. 2011;185(1):132–137.
- Ko J, Falzarano SM, Walker E, et al. Prostate cancer patients older than 70 years treated by radical prostatectomy have higher biochemical recurrence rate than their matched younger counterpart. Prostate. 2013;73(8):897–903.
- Vukovic M, Kavaric P, Magdelinic A, et al. Perineural invasion on biopsy specimen as predictor of tumor progression in aging male treated with radical prostatectomy. Could we use it for pre-surgical screening? Aging Male. 2019;1–6. DOI:10.1080/13685538.2019.1581758
- Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2016. Bethesda (MD): National Cancer Institute. Available from: https://seer.cancer.gov/csr/1975_2016/.
- Encuesta Nacional de Salud y Nutrición Cuernavaca (ENSANUT). 2012. México: Instituto Nacional de Salud Pública. https://ensanut.insp.mx/encuestas/ensanut2012/index.php.
