Abstract
Objectives
The beneficial effects of testosterone replacement therapy (TRT) in men with late-onset hypogonadism (LOH) on the body composition and metabolic outcomes are well-established. A potential explanation might lie in the hormones, secreted from skeletal muscles, named “myokines". The aim of this study was to evaluate the effects of TRT on the levels of serum irisin in subjects with LOH.
Study Design
A total 40 men with metabolic syndrome (MS) and LOH (measured serum testosterone concentration < 12 nmol/l). TRT with Testosterone Undecanoate (Nebido™) was performed at baseline and at week 6. Irisin serum concentration was determined at baseline and at week 18 by means of ELISA.
Results
Circulating irisin was positively associated with serum testosterone (r = 0.283, p < 0.05). TRT has led to a statistically significant rise in circulating serum irisin levels (7.12 ± 0.76 mcg/ml versus 7.76 ± 0.75 mcg/ml; paired-samples t-test p < 0.001). ROC-analyses determined irisin to be predictive of treatment response (AUC = 0.741, p = 0.014).
Conclusions
Irisin is positively associated with serum testosterone in a population of men with MS and LOH. TRT in these subjects has led to a significant improvement in associated clinical symptoms as well as to a significant rise in serum irisin levels.
1. Introduction
Male hypogonadism is a clinical syndrome that occurs due to a failure of the testis to produce adequate amounts of testosterone and/or impairment of the spermatogenesis [Citation1]. It is an increasingly common condition – as 38.7% of men aged 45 and older are reported to fulfill the diagnostic criteria [Citation2]. Late-onset hypogonadism (LOH), on the other hand, is defined by decreasing circulating testosterone concentrations, occurring in middle-aged and elderly men and is characterized by low serum testosterone and low or normal gonadotropin levels [Citation3]. It typically presents with decreased libido, erectile dysfunction, increased fat mass and reduced muscle mass [Citation4,Citation5]. More recently, the term “functional hypogonadism” has been used to describe the presence of repeatedly low serum testosterone concentrations after excluding organic causes of hypogonadism [Citation6]. According to the current guidelines, population screening for male hypogonadism is not recommended, but subjects with type 2 diabetes mellitus (T2DM), obesity, infertility, osteoporosis, chronic obstructive pulmonary disease, and HIV should be actively screened [Citation6,Citation7].
Metabolic syndrome (MS) is a major risk factor for the development of T2DM [Citation8] and its components are commonly found in men with hypogonadism [Citation9–12]. Furthermore, testosterone deficiency was suggested to have a causal relationship with the locomotive syndrome – a condition associated with sarcopenia that leads to difficulties in movement and to an impaired quality of life [Citation13].
The beneficial effects of testosterone on the body composition and on the cardio-metabolic estimates are well-established [Citation14]. Testosterone replacement in deficient individuals ameliorates metabolic parameters [Citation15], improves vascular function [Citation16], and reduces body weight [Citation17]. Finally, it has been shown that long-term testosterone therapy improves urinary sexual function and QoL in men with hypogonadism [Citation18].
In a previous publication, we speculated that a potential explanation for these effects might lie in the concept that muscles produce humoral factors of that have various effects on other systems [Citation19]. These hormones, named “myokines” [Citation20,Citation21] might mediate the positive effects of testosterone on the human metabolism, or conversely, might explain the deterioration of the metabolic profile in men with hypogonadism [Citation22]. Irisin is one of the most studied myokines since it is discovery [Citation23]. It has been shown that it promotes energy expenditure by means of browning of white adipose tissue [Citation24]. Thus, it has been suggested that it acts as a transmitter of the beneficial effects of physical activity on adipose tissue and the metabolism [Citation25]. In cross-sectional studies, irisin correlates well with parameters of the glucose/lipid metabolism and may prevent diabetes and obesity [Citation26,Citation27]. However, no prospective studies in humans have been performed to shed further light on this potential association.
Numerous interventional TRT studies have shown beneficial effects of testosterone on various parameters of the cardiometabolic syndrome [Citation28–30]. The aim of this proof of concept observational prospective study, however, was to evaluate the impact of testosterone treatment on serum irisin as a potential mediator of these effects in a population of men with LOH and MS.
2. Materials and methods
2.1. Study design
This was an observational prospective single-center study to assess the impact of testosterone replacement therapy /TRT/ with testosterone undecanoate /Nebido®/ on various metabolic and clinical outcomes and serum irisin levels in men with LOH and MS.
2.2. Study population
A total of 40 men recruited in a university hospital participated in the study.
Inclusion criteria were as follows /all criteria were necessary/:
Presence of LOH /measured 7:00 AM to 11:00 AM serum testosterone < 12 nmol/l/ [Citation6,Citation31] on two separate days and associated clinical symptoms, evaluated by ADAM questionnaire
MS (3 of 5 criteria), according to the IDF criteria as follows: (1) abdominal obesity, defined as waist circumference ≥94 cm; (2) elevated blood pressure, defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, or current antihypertensive drug treatment; (3) elevated fasting blood glucose level ≥5.6 mmol/l or current use of blood glucose lowering agents or history/diagnosis of T2DM; (4) decreased HDL cholesterol level <1.03 mmol/or drug treatment aimed to increase HDL cholesterol; and (5) hypertriglyceridemia ≥1.70 mmol/l or drug treatment for elevated triglycerides [Citation8].
Age 45–74 years.
Signed informed consent.
Exclusion criteria were as follows: previous major adverse cardiovascular event (stroke, myocardial infarction, stenting procedure) in the past 6 months; liver dysfunction (any hepatic enzyme >3 times the upper-limit of normal); chronic kidney disease (eGFR estimated by CKD-EPI <60 ml/min/1.73m2); neoplastic disease; congestive heart failure – NYHA class III or higher; prostate-specific antigen ≥4 ng/ml or a suspicion of a prostate neoplasia; hematocrit >50%.
2.3. Anthropometric measurements
All anthropometric measurements were performed by a single medical professional. They included waist circumference, weight, height, body mass index (BMI), arterial blood pressure. BMI was calculated as weight in kilograms divided by height squared in squared meter. Waist circumference was measured at the midpoint between the inferior costal margin and the superior border of the iliac crest on the mid-axillary line. Body composition was determined by means of body impedance analysis – Tanita™ MC780 MA.
2.4. Biochemical measurements
Serum irisin was estimated by means of a commercially available enzyme-linked immunosorbent assay kit (ELISA, BioVendor™, Czech Republic). Sensitivity threshold reported to be 1 mcg/ml, detectability range 0.001–5 mcg/ml, CV = 4.9–9.7%. Fasting blood samples were obtained between 7:00 and 11:00 AM to measure serum total testosterone, estradiol, luteinizing hormone (LH), prolactin, using an electrochemiluminescent method.
2.5. Questionnaires
The International Index of Erectile Function-5/IIEF-5/ [Citation32] and Androgen Deficiency in the Aging Male/ADAM/ [Citation33] questionnaires were performed on all participants before and after the intervention.
2.6. Intervention
Subjects enrolled in the study received intramuscular injections of testosterone undecanoate /Nebido™/ 1000 mg at baseline /week 0/ and week 6. Control of biochemical and clinical parameters as well as serum irisin concentration measurement was performed at week 18. Intervention is considered to be standard-of-care as it is following the established guidelines for TRT in the general population. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The study has been approved of the local ethics committee. All subjects signed an informed consent form.
2.7. Statistical analysis
Data were presented as means (standard deviation) for continuous variables or numbers (percentages) for categorical variables. Nonparametric Kolmogorov-Smirnov and Shapiro-Wilk tests were used for the determination of distribution. Paired samples T-test was used for the comparison of various parameters pre- and post-TRT. ROC-analysis was performed for the evaluation of significance of serum irisin for the determination of treatment response. Backwards univariate linear regression was used to estimate the predictive value of circulating irisin for the response to TRT. Data were analyzed by statistical package SPSS Statistics™ v. 21.0 (IBM™).
3. Results
Baseline characteristics of the study population are shown on . A total of 40 men with established MS and LOH participated in the study. Mean ± SD age of the study participants was 49.4 ± 4.1. Mean ± SD BMI was 39.9 ± 10.2. 50% of the study participants had newly diagnosed T2DM, 30% had previous history of cardiovascular disease, 70% had dyslipidemia and 90% were hypertensive. As serum testosterone is impacted by concomitant neoplastic, inflammatory, cardiovascular, or decompensated metabolic conditions [Citation34–36], subjects were excluded from the study if any of the aforementioned were present. Inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate were within reference range in all subjects.
Table 1. Baseline characteristics of the study population.
Spearman correlation analyses determined that circulating serum irisin is positively associated with serum testosterone (r = 0.283, p < 0.05) and fat-free mass as determined by BIA (r = 0.370, p < 0.05) and negatively with hepatic enzymes (ASAT, ALAT) and serum uric acid (r = –0.257, p < 0.05). Results from the correlation analyses can be seen on . Serum testosterone correlated negatively with BMI (r = –0.470, p < 0.01), waist circumference (r = –0.352, p < 0.05), and serum triglycerides (r = –0.447, p < 0.01), and positively with IIEF-5 questionnaire score (r = 0.566, p < 0.01).
Table 2. Spearman correlations of circulating serum irisin (n = 40) with some anthropometric and biochemical parameters of the study population.
Paired-samples T-test was performed to establish whether the treatment had an effect on various anthropometric and biochemical parameters as well as circulating serum irisin concentration. Results of the multiple paired-samples t-tests can be seen on . Following the TRT, subjects reduced their BMI (p < 0.026), waist circumference (p < 0.001), and improved ADAM (p < 0.001) and IIEF-5 (p = 0.007) questionnaire scores significantly and increased their testosterone (p < 0.001) and serum irisin concentration (p < 0.001). No significant changes in the body composition as measured by BIA were observed following the intervention. As a result of the TRT intervention, 14 subjects normalized serum testosterone levels (>12 mmol/l), while 26 remained below the threshold. We have performed multiple ROC-analyses to determine the predictive value of various baseline parameters (age, BMI, baseline questionnaire scores, baseline testosterone concentration) for treatment response, defined as normalization of testosterone levels, significant improvement in ADAM score and/or improvement in body composition. Of note, baseline irisin concentration has had similar predictive value (AUC = 0.741, p = 0.014, 95% CI 0.571–0.911) for treatment response as baseline testosterone concentration (AUC = 0.744, p = 0.013, 95% CI 0.588–0.900; ). Finally, regression analysis determined that circulating baseline irisin level of ≥7.13 mcg/ml was associated with an odds ratio of 3.309 (95%CI 1.099–9.065, p = 0.033) for predicting a normalization of serum testosterone levels and improvement in ADAM questionnaire score in our study population.
Figure 1. ROC-curves for the determination of the predictive value of Age (AUC = 0.448 95% CI 0.251–645, p = 0.596); Baseline BMI (AUC = 0.467 95% CI 0.275–659, p = 0.739); Baseline Serum Irisin (AUC = 0.741, 95% CI 0.571–0.911, p = 0.014); Baseline Serum Testosterone (AUC = 0.744 95% CI 0.588–0.900, p = 0.013) for treatment response – normalization of serum testosterone following the intervention.
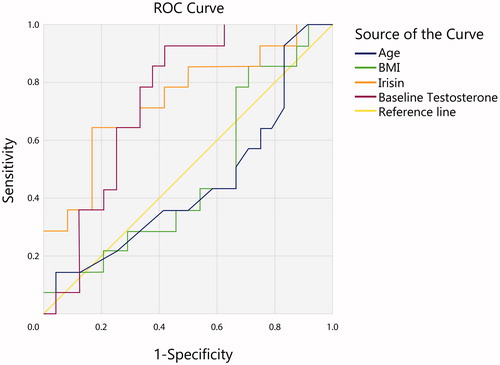
Table 3. Various biochemical and anthropometric parameters of the study population at baseline and at week 18, following the intervention of TRT (Testosterone Undecanoate 1000 mg IM at baseline and at week 6).
4. Discussion
In this prospective observational study, we evaluated the impact of testosterone replacement therapy on various cardio-metabolic estimates and on circulating irisin levels in a population of men with LOH and MS.
Various studies have shown that circulating testosterone is inversely associated with BMI, WC, and TC in adult men [Citation9,Citation10,Citation37]. Furthermore, testosterone replacement has been shown to ameliorate these parameters and improve quality of life [Citation38–41]. Similarly, in our study, serum testosterone correlated negatively with traditional estimates of cardio-metabolic risk and obesity estimates. Additionally, testosterone treatment has led to a significant decrease in BMI and waist circumference and an improvement in ADAM and IEEF-5 scores which are reflective of quality of life. In our study, albeit of short duration, no safety concerns of testosterone therapy were raised.
In a previous report by our group, we have shown that irisin levels were found to be negatively associated with circulating serum testosterone in a cohort of men with MS and T2DM [Citation19]. This has suggested a potential involvement of irisin in the pathogenesis of LOH and has prompted further prospective research. In the current study, however, a positive correlation between serum testosterone and irisin was observed, whilst TRT intervention has led to a statistically significant rise in serum irisin concentrations. Based on a previously suggested mechanism, we speculate, that these observed differences between the two studies might be due to a potential “irisin resistance” which is owed to a possible discordant regulation between irisin and the metabolism in subjects with long standing diabetes [Citation42]. In the current study, subjects were either non-diabetic or with new-onset diabetes, whereas in the previous cross-sectional study, mean diabetes duration was 6.1 years which might explain the observed differences. Furthermore, subjects in the previous study were significantly older, had significantly higher testosterone concentrations and were significantly less obese. As adiposity has also been suggested to contribute to circulating irisin levels [Citation43], it could also explain some of the discrepancies in the two studies.
In another study by our team, irisin was found to correlate negatively with traditional metabolic parameters such as FPG, hepatic enzymes, and insulin in high risk individuals, and to decrease with worsening of the carbohydrate tolerance [Citation44]. Negative correlations between circulating irisin and hepatic enzymes were also observed in the current prospective study.
Several animal model interventional studies shed some light on the potential involvement of androgens and serum irisin. A study in obese female BALB/c mice models has evaluated the impact of injected high-dose irisin on serum testosterone concentration but found no significant difference following the intervention [Citation45]. Another interventional study in mice evaluated the impact of intracerebrovascular administration of irisin on the hypothalamus-pituitary-gonadal axis and found a decrease in serum testosterone, LH, and FSH levels [Citation46]. Lastly, one study has shown that TRT in orchiectomized rats leads to a statistically significant decrease in serum irisin [Citation47].
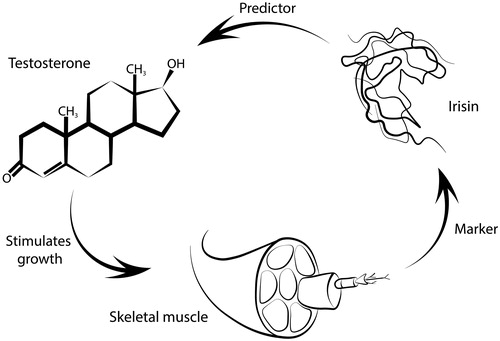
As interventional studies on the potential impact of TRT on irisin concentration in humans are lacking, the intimate mechanisms of this interrelations are still unclear. We speculate that the anabolic effects of testosterone might lead to an increase of irisin concentration by means of an increase in fat-free mass. Thus, irisin concentration could be reflective of baseline muscle mass and could, therefore predict treatment response of TRT, as higher baseline fat mass could be associated with higher aromatization ().
Figure 2. Suggested mechanism of interaction between testosterone, irisin, and striated muscles. Irisin acts as a marker for muscle tissue and in thus could potentially be used as a predictor for treatment response of TRT.
The limitations of our study include relatively small sample size as well as a lack of a control group. Selection bias could also be suspected as subjects were deliberately chosen to be of a high-risk population for the development of metabolic complications in a university hospital setting.
5. Conclusions
As a conclusion, our proof-of-concept study reveals a potential causal relationship between irisin and testosterone in humans. We believe, that on the basis of this data, further, prospective placebo-controlled trials are required to further elucidate the impact of myokines on the human metabolism.
Author contributions
YA: I declare that I participated in the collection of samples, statistical analysis and writing of the manuscript and that I have seen and approved the final version. I have no conflict of interest. AG: I declare that I participated in the collection of samples, statistical analysis and review of the manuscript and that I have seen and approved the final version. I have no conflict of interest. VK: I declare that I participated in the collection of samples, statistical analysis and review of the manuscript and that I have seen and approved the final version. I have no conflict of interest. TG: I declare that I participated in the collection of samples, statistical analysis and reviewing of the manuscript and that I have seen and approved the final version. I have no conflict of interest. IN: I declare that I participated in the collection of samples, statistical analysis and writing of the manuscript and that I have seen and approved the final version. I have no conflict of interest. TV: I declare that I participated in the collection of samples, performing ELISA methods, review of the manuscript and that I have seen and approved the final version. I have no conflict of interest. ZK: I declare that I participated in the collection of samples, statistical analysis and review of the manuscript and that I have seen and approved the final version. I have no conflict of interest.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Corona G, Rastrelli G, Maggi M. Diagnosis and treatment of late-onset hypogonadism: systematic review and meta-analysis of TRT outcomes. Best Pract Res Clin Endocrinol Metab. 2013;27(4):557–579.
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762–769.
- Bassil N. Late-onset hypogonadism. Med Clin North Am. 2011;95(3):507–523.
- Kirby M. Testosterone deficiency. Pract Nurse. 2015;45:32–36.
- Almehmadi Y, Yassin DJ, Yassin AA. Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism. Aging Male. 2015;18(3):186–194.
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559.
- Lunenfeld B, Mskhalaya G, Kalinchenko S, et al. Recommendations on the diagnosis, treatment and monitoring of late-onset hypogonadism in men - a suggested update. Aging Male. 2013;16(4):143–150.
- Eckel R, Alberti K, Grundy S, et al. The metabolic syndrome. Lancet. 2010;375(9710):181–183.
- Goh VH, Hart WG. Association of general and abdominal obesity with age, endocrine and metabolic factors in Asian men. Aging Male. 2016;19(1):27–33.
- Rotter I, Kosik-Bogacka D, Dołęgowska B, et al. Analysis of relationships between the concentrations of total testosterone and dehydroepiandrosterone sulfate and the occurrence of selected metabolic disorders in aging men. Aging Male. 2015;18(4):249–255.
- Yassin AA, Saad F, Gooren LJ. Metabolic syndrome, testosterone deficiency and erectile dysfunction never come alone. Andrologia. 2008;40(4):259–264.
- Dean JD, McMahon CG, Guay AT, et al. The International Society for Sexual Medicine's process of care for the assessment and management of testosterone deficiency in adult men. J Sex Med. 2015;12(8):1660–1686.
- Fink JE, Hackney AC, Matsumoto M, et al. Mobility and biomechanical functions in the aging male: testosterone and the locomotive syndrome. Aging Male. 2018;29:1–8.
- Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91.
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf). 2016;84(1):107–114.
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21(3):158–169.
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3(3–4):73–83.
- Haider KS, Haider A, Doros G, et al. Long-term testosterone therapy improves urinary and sexual function, and quality of life in men with hypogonadism: results from a propensity matched subgroup of a controlled registry study. J Urol. 2018;199(1):257–265.
- Kamenov Z, Assyov Y, Angelova P, et al. Irisin and testosterone in men with metabolic syndrome. Horm Metab Res. 2017;49(10):755–759.
- Henningsen J, Rigbolt KT, Blagoev B, et al. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9(11):2482–2496.
- So B, Kim HJ, Kim J, et al. Exercise-induced myokines in health and metabolic diseases. Integr Med Res. 2014;3(4):172–179.
- Schiaffino S, Dyar K, Ciciliot S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–4314.
- Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468.
- Rodriguez A, Becerril S, Ezquerro S, et al. Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf). 2017;219(2):362–381.
- Arhire LI, Mihalache L, Covasa M. Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2019;10:524.
- Yan B, Shi X, Zhang H, et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS One. 2014;9(4):e94235.
- Tang L, Tong Y, Zhang F, et al. The association of circulating irisin with metabolic risk factors in Chinese adults: a cross-sectional community-based study. BMC Endocr Disord. 2019;19(1):147.
- Yassin A, Haider A, Haider KS, et al. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Diabetes Care. 2019;42(6):1104–1111.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186–1192.
- Hackett G, Cole N, Mulay A, et al. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int. 2019;123(3):519–529.
- Dimopoulou C, Ceausu I, Depypere H, et al. EMAS position statement: testosterone replacement therapy in the aging male. Maturitas. 2016;84:94–99.
- Mohamed O, Freundlich RE, Dakik HK, et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res. 2010;22(1):20–24.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830.
- Erenpreiss J, Fodina V, Pozarska R, et al. Prevalence of testosterone deficiency among aging men with and without morbidities. Aging Male. 2019;1:1–5.
- Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019; 22(2):129–140.
- Angelova P, Kamenov Z, Tsakova A, et al. Interleukin-18 and testosterone levels in men with metabolic syndrome. Aging Male. 2018;21(2):130–137.
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male. 2014;17(3):161–165.
- Almehmadi Y, Yassin AA, Nettleship JE, et al. Testosterone replacement therapy improves the health-related quality of life of men diagnosed with late-onset hypogonadism. Arab J Urol. 2016;14(1):31–36.
- Yassin DJ, Doros G, Hammerer PG, et al. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–1576.
- Salman M, Yassin DJ, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20(1):45–48.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19(1):64–69.
- Al-Daghri NM, Alokail MS, Rahman S, et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur J Clin Invest. 2015;45(8):775–781.
- Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–E778.
- Assyov Y, Gateva A, Tsakova A, et al. Irisin in the glucose continuum. Exp Clin Endocrinol Diabetes. 2016;124(01):22–27.
- Majeed S, Shafi R, Moin H, et al. Effects of recombinant irisin on body mass index, serum insulin, luteinizing hormone and testosterone levels in obese female BALB/c mice. J Coll Physicians Surg Pak. 2019;29(08):736–740.
- Tekin S, Beytur A, Erden Y, et al. Effects of intracerebroventricular administration of irisin on the hypothalamus-pituitary-gonadal axis in male rats. J Cell Physiol. 2019;234(6):8815–8824.
- Doulamis IP, Tzani A, Konstantopoulos P, et al. Experimental hypogonadism: insulin resistance, biochemical changes and effect of testosterone substitution. J Basic Clin Physiol Pharmaco. 2019;4(3):30.
