Abstract
Introduction
Neutrophil-to-lymphocyte (NLR) and platelet-to-lymphocyte ratios (PLR) are useful clinical biomarkers for prognosis in several malignancies. Their predictive value has been less clearly demonstrated with prostate cancer (PCa), particularly, their utility within active surveillance (AS) protocols. We aim to evaluate NLR and PLR in AS patients.
Methods
We identified 98 patients who met inclusion criteria in our cohort of 274 men diagnosed with PCa on AS. Patients were then categorized into high and low NLR and PLR groups.
Results
The 2.5 and 5-year Gleason upgrading free probability for our high NLR cohort was 73.9%(CI 56.3% to 97.0%) and 46.2%(CI 22.4% to 95.1%) compared to 76.3%(CI 65.7% to 88.7%) and 61.7%(CI 47.7% to 80.0%) in the low NLR cohort(p = .73). The 2.5 and 5-year Gleason upgrading free probability for our High PLR cohort was 73.5%(CI 57.3% to 94.2%) and 60.1(CI 41.4% to 87.4%) compared to 76.8%(CI 65.8% to 89.65) and 58.1%(CI 42.2% to 80.1%) in our low PLR group(p = .41). A multivariant analysis demonstrated these groups were not significant predictors of upgrading or treatment.
Conclusion
Despite their usefulness in many types of malignancy, NLR and PLR were not predictors of upgrading or treatment in men on AS for localized PCa in our cohort.
Introduction
Active surveillance (AS) offers prostate cancer (PCa) patients with low-grade disease the option to defer potentially adverse interventions in favor of observation and serial monitoring. It remains the preferred treatment option for patients with low-risk disease. A recent study published by Mahal et al. demonstrated that the percentage of men electing for AS or watchful waiting increased from 14.5% in 2010 to 42.1% in 2015 [Citation1]. As the number of men electing for AS increases, the need for objective markers to help guide treatment decisions also increases. While utilization of genomic testing and multiparametric magnetic resonance imaging tests have been introduced into this space, further determination with biologically active markers without the expense of those tests would be of benefit to patients.
Appropriate patient selection is crucial when identifying candidates for active surveillance. Key elements in this process are prompt diagnosis and adequate disease staging. The advent of prostate specific antigen (PSA) testing in the late 1980s, followed by the widespread use of PSA for screening purposes in the 1990s revolutionized the detection and timely treatment of PCa [Citation2]. PSA testing offers strong utility for screening, diagnostic, and therapeutic management of PCa patients, however, there are limitations to its use and major concerns exist regarding its role in overdiagnosis and overtreatment of the disease [Citation3,Citation4]. These limitations leave prostate cancer researchers searching for novel tools to adequately counsel patients on screening and treatment strategies for PCa. As an adjunct, advanced imaging techniques utilizing multiparametric magnetic resonance imaging (MRI) have become a mainstay in clinical urology due to their ability to effectively diagnose clinically significant prostate cancer [Citation5]. However, MRI testing can be costly, and while it has significantly bolstered the clinical arsenal against PCa, but it still leaves room for less costly clinical detection.
The search for comprehensive PCa risk stratification has taken many forms. Some authors have sought to identify existing comorbidities that may be associated with PCa as a means of risk stratifying disease detection. Ohwakia and colleagues found an associated between incidental high-risk prostate cancer diagnosis and pre-existing diabetes mellitus in a cohort of men undergoing holmium laser enucleation (HoLEP) for treatment of benign prostatic hyperplasia (BPH)[Citation6]. Other researchers have promoted the study of molecular pathways leading to prostate cancer diagnosis and management. Yu et al. studied the frequency of mitochondrial DNA (mtDNA) mutations in PCa diagnoses and found that 75.5% of prostate cancer biopsy specimens exhibited mtDNA deletions compared to only 14.7% of BPH tissue [Citation7]. These researchers also found mtDNA mutations demonstrated statistical significance with higher Gleason scores compared to specimens without these mutations. This study suggests mtDNA mutations could be useful biomarkers for prostate cancer prognosis. Furthermore, Terrealba and colleagues analyzed the utility of immunohistochemical staining within post-radical prostatectomy prostate cancer specimens and the prognostic roles of molecular markers. This group found evidence of an association between PI3K expression and biochemical disease progression in PCa [Citation8]. These findings underscore the importance of research into biomarkers as fundamental to the future of prostate cancer treatment. Most clinicians and researchers agree, though, that inexpensive, minimally invasive testing is the ideal combination for a useful clinical biomarker – making neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) highly desirable candidates.
The value of NLR and PLR are based in their relationship to chronic inflammatory changes. Chronic inflammation has long been suspected as a driving factor in the development of malignancy. More recently, a growing body of evidence has substantiated the suspicion that systemic inflammatory responses contribute to cancer development and progression [Citation9]. This understanding has fueled interest in using basic hematologic parameters to diagnose and treat various solid tumor malignancies. NLR and PLR have proven to be both diagnostic and prognostic clinical biomarkers for several malignancies, including pulmonary, colorectal, and renal [Citation10,Citation11]. Research suggests that neutrophils may promote tumor development and metastasis through cytokine signaling while platelets may act by release of platelet-derived growth factor and transforming growth factor [Citation12,Citation13]. Furthermore, racial differences in cancer-related immunologic regulation is an active and growing field of study. In a comprehensive review article evaluating racial differences in immunologic regulation, King Thomas and colleagues outline the importance of differences in cytokine expression profiles and steroid hormone pathways that likely contribute to cancer specific outcome variations among AA men [Citation14]. The racial disparities in prostate cancer diagnosis, disease progression, and outcomes has been demonstrated in the literature [Citation15,Citation16]. Although these outcome disparities are undoubtedly multifactorial, a better understanding of immunological profiles is essential in discovering clinically significant biomarkers such as NLR and PLR in racially diverse populations.
Significant literature does exist on the hematologic parameters among prostate cancer patients. Multiple studies have noted the importance of NLR and PLR in high risk and metastatic disease, however, the predictive values of NLR and PLR have been less clearly demonstrated with low-risk localized PCa [Citation17–19]. Particularly, their utility in low-risk patients electing AS has been poorly defined. Our study aimed to analyze the value of NLR and PLR as additional prognostic factors in predicting disease upgrading and eventual need for treatment in the AS PCa population.
Methods
We identified all men on a prospective observational study for AS at the South Louisiana Veterans Administration Medical Center (SLVHCS), New Orleans, LA which includes consecutive patients enrolled from 2013 to present. Generally, all patients diagnosed with low or very-low-risk PCa are encouraged to pursue AS regardless of the volume of disease or age of patient. For this study, we queried our total cohort of 274 patients diagnosed with PCa electing AS. For subset analysis, we restricted the inclusion criteria to individuals who had completed at least two prostate biopsies; a diagnostic biopsy and then a confirmatory biopsy generally performed within the first year of diagnosis and complete blood count workup within three months of their first biopsy, without evidence of an acute infection at the time of hematologic evaluation. In total, 98 men met inclusion and were entered into this study.
Patient management
For men managed at the SLVHCS electing AS, PSA was performed every six months with annual DRE examinations. A confirmatory biopsy was performed within 12 months of the initial biopsy and then repeated every two to three years or for-cause, which was defined as a rapid change in PSA, change in physical exam, or new radiographic findings. All patients had a 14-core biopsy on diagnostic testing. Each confirmatory biopsy ranged from 12- to 22-cores based on MRI findings. Beginning in 2017, we performed MRI fusion biopsy for confirmatory biopsy. Gleason progression was defined as pathologic upgrading of Gleason score above diagnostic Gleason score. Men who did not present with Gleason progression continued AS while those who upgraded were recommended definitive treatment. Most patients with Gleason upgrading elected radical treatment in the form of radical prostatectomy or radiation therapy. Some elected to continue AS; if a patient upgraded but elected to remain on AS, he was included in treatment and overall survival (OS) analyses but failed in the upgrading analyses.
Statistical methods
Patient’s clinical, pathology, and laboratory data were collected and correlated to different outcome variables including Gleason progression and treatment. Using R version 3.5.3 packages “survival” and “survminer,” Kaplan–Meier upgrading-free probability and treatment-free probability plots were created. The Log-rank p value was used to determine difference among groups. Hazard Ratios (HR) were represented through multivariate Cox proportional hazards regression analyses, performed using R package “survivalAnalysis.”
Results
Patient demographics
At SLVHCS, 274 men were identified on AS for PCa, 98 men met the inclusion criteria and were entered into this study for statistical analysis; this included 66 African American (AA) and 32 Caucasian (CA) subjects. Neutrophil Lymphocyte Ratio (NLR) was divided into two groups: High and Low. High NLR was defined as one standard deviation above mean of our cohort. Low NLR or PLR was defined as less than one standard deviation above the mean of our cohort. 74 patients were entered into the Low NLR group with 24 in the High NLR group. In a separate analysis, 73 were denoted Low PLR group and 25 sorted into the High PLR group.
Complete demographic data is present in . The median age at enrollment in AS was 67.2 years old. High and Low NLR and PLR patients had similar ages of diagnoses and median BMI (NLR: p = .97, PLR: p = .09). When comparing PSA density, prostate volume, number of positive cores, and baseline testosterone level at first and second biopsy among the High and Low NLR and PLR men, the groups appeared statistically similar (). As expected, High and Low NLR groups showed a statistically different NLR value at 5.15 and 2.00, respectively (p < .0001). Additionally, High and Low PLR groups showed statistically different PLR value at 253.9 and 118.7, respectively (p < .0001). Patients in this study were on AS protocol for an average of 2.56 years with High and Low NLR patients being monitored for an average of 3.0 and 2.4 years respectively (p = .27). High and Low PLR patients were monitored for an average 2.5 and 2.6 years respectively (p = .80). The majority of patients in both cohorts showed no evidence of upgrading or treatment at the time of the data lock. No patients in this cohort demonstrated metastasis or death from PCa over the course of study.
Table 1. Patient demographics for our PLR and NLR groups. High laboratory value in both groups showed a statistically significant difference in their respective values. All other demographic values were comparable between High and Low groups for both hematologic parameters. The total number of patients of 98 went through two separate analysis, NLR and PLR.
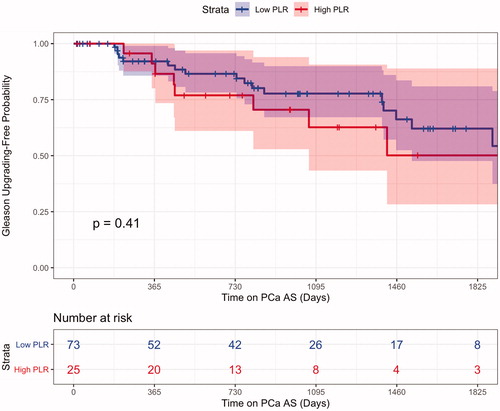
Gleason upgrading and treatment
Over the entirety of the study, a total of 25 men upgraded from their initial diagnosis. The 2.5- and 5-year Gleason upgrading free probability for the whole cohort was 75.6% (CI 66.3% to 86.3%), and 58.7% (CI 46.0% to 75.0%), respectively. For the High NLR cohort, the 2.5- and 5-year Gleason upgrading free probability was 76.3% (CI 65.7% to 88.7%) and 61.7% (CI 47.7% to 80.0%), respectively. For the Low NLR cohort, the 2.5- and 5-year Gleason upgrading free probability was 76.3% (CI 65.7% to 88.7%) and 61.7% (CI 47.7% to 80.0%), respectively. The Gleason upgrading log-rank p value was calculated 0.73 between the two NLR groups (). The 2.5- and 5-year Gleason upgrading free probability for our High PLR cohort was 73.5% (CI 57.3% to 94.2%) and 60.1 (CI 41.4% to 87.4%) compared to 76.8% (CI 65.8% to 89.65) and 58.1% (CI 42.2% to 80.1%) in our Low PLR group (p = .41) ().
Figure 1. NLR Kaplan–Meier curve for pathologic upgrading showed men with increased NLR values, at the time of diagnosis of prostate cancer, were not seen to upgrade at higher overall rates then their lower NLR counterparts.
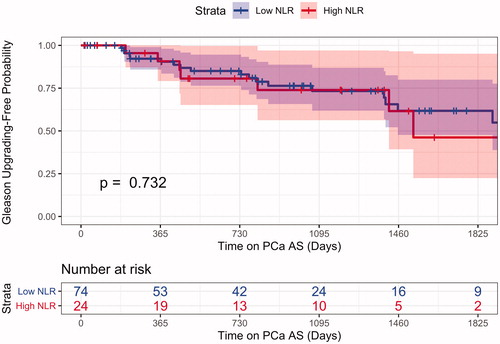
Figure 2. PLR Kaplan–Meier curve for pathologic upgrading for patients presenting with high PLR laboratory values at the time of diagnosis show comparable overall Gleason upgrading rates on subsequent biopsy.
Of the initial 98-patient cohort, 38 (39%) men elected treatment and were considered AS failures. The most likely cause of AS failure was Gleason Upgrading (25 patients, 7 High and 18 Low NLR separately 8 High and 17 Low PLR) followed by elective treatment (13 patients, 6 High and 7 Low NLR separately 4 High and 9 Low PLR). No differences in NLR or PLR groups were seen between the treatment groups or indication for AS failure (p = .46).
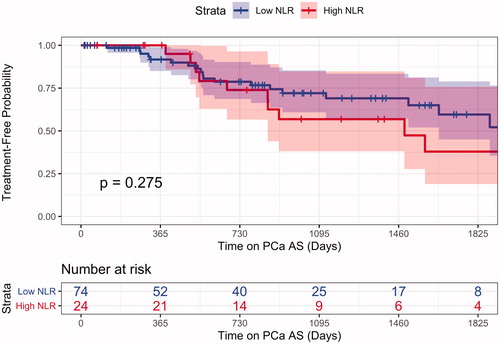
The 2.5- and 5-year treatment-free probability for the entire cohort was 69.4% (CI 59.4% to 81.1%) and 53.4% (CI 40.8% to 69.9) respectively. For our High NLR group the 2.5- and 5-year treatment-free probability was 56.8% (CI 38.1% to 84.8%) and 37.9% (CI 19% to 75.8%), respectively. Our Low NLR group had a 2.5- and 5-year treatment-free probability of 74.4% (CI 63.5% to 87.1%) and 59.6% (CI 45% to 78.9%), respectively. When comparing the treatment difference between the two NLR groups, the log-rank p = .27 (). Treatment-free probability showed similar results in our PLR groups with a non-significant p value.
Figure 3. NLR Kaplan–Meier curve for treatment showed comparable overall rates of treatment between men who presented with high and low NLR values at the time of diagnosis.
In a multivariate Cox proportional hazards regression analysis, only one variable showed significance to predict Gleason upgrading, more than two positive cores at diagnostic biopsy HR 5.90 (CI 1.67–20.91) p = .0059. High NLR and PLR groups were not significant, HR 1.70 (CI 0.75–9.83) p = .55 and 0.24 (CI 0.03–2.31) p = .22, respectively ().
Table 2. Multivariate cox regression analysis for the hematologic parameters we evaluated in this study, PLR and NLR, we not significant predictors of pathologic upgrading on AS. An increased number of positive biopsy cores >2 on diagnosis, was seen to be a predictor of Gleason upgrading for patients on an AS protocol. Additionally, no other factors were seen to be prognostic predictors of progression.
Discussion
Clinicians use a variety of clinical, pathological and laboratory data to assist in selecting patients who are good candidates for AS. Vukovic and colleagues have recently demonstrated a correlation between perineural invasion on prostate biopsy as a means of conveying worse prognostic outcomes for PCa patients [Citation20]. In our study, we aimed to evaluate whether NLR and or PLR provide additional support to clinicians when counseling patients on the nature of their PCa diagnosis and their likelihood of seeing disease progression. By identifying NLR and PLR as limited value prognostic indicators, our study adds value to our current understanding of AS. We believe this helps clinicians narrow focus to valuable clinical guides outside of these hematologic parameters.
38 out of 98 (39%) patients ultimately underwent definitive management for their PCas, which is in line with previously published AS treatment rates [Citation21]. None of NLR or PLR categories we analyzed showed any statistical significance in predicting upgrading of PCa diagnosis or the ultimate need for definitive treatment of the patient’s cancer. We did find that the number of positive cores at diagnostic biopsy was statistically significant for upgrading which is a finding that has been demonstrated prior to this article [Citation22].
Multiple studies have demonstrated a positive correlation between NLR and PLR and the staging of various cancers [Citation23,Citation24]. This includes patients with genitourinary malignancies such as renal or bladder cancer. This relationship has also been demonstrated with metastatic PCa. Peng et al. performed a meta-analysis of 32 studies of pre-treatment NLR and PLR values in PCa patients and found that NLR is a prognostic predictor of shorter OS in metastatic castrate resistant prostate cancer (mCRPC) [Citation25]. They theorize – as other studies have – that an elevated neutrophil count may promote metastatic tumor potential via cellular signaling pathways that suppress anti-tumor activity and promote migratory and invasive potential in malignant cells. The relationship with PLR was less clearly defined in this study. However, Wang et al. performed a meta-analysis on the prognostic role of PLR in PCa using six studies including 1324 patients and demonstrated a correlation between overall-survival and disease-free survival [Citation26]. This result included combined data on localized and metastatic disease.
In this cohort, we did not find the correlation between the hematologic parameters examined and the likelihood of treatment or Gleason upgrading following AS. It is likely that the low volume of disease and low-grade status of these patients’ tumors limits the utility of NLR and PLR data. Ferro and colleagues analyzed NLR and PLR in a retrospective study of 260 patients who ultimately underwent radical prostatectomy, and were eligible for AS based on low-risk disease parameters. Their findings did conclude NLR and PLR were predictors of Gleason upgrading [Citation27]. This study population differed from this paper’s in that it was a retrospective study in patients who had definitive treatment among a more racially homogenous group of Italian men. Other groups have analyzed the utility of NLR in low-risk PCa with some conflicting results. In a retrospective analysis of 210 low-risk PCa patients who underwent radical prostatectomy, Gokce et al. demonstrated that NLR is not a predictor of disease upstaging in patients with low-risk PCa, but it can be a predictor of Gleason score upgrading and biochemical recurrence [Citation28]. In contrast, Kwon et al. found NLR was not associated with upgrading, upstaging, or biochemical recurrence (BCR) in their retrospective study of 217 low-risk PCa patient who met AS criteria and underwent robot-assisted radical prostatectomy. Sun et al. found PLR to be associated with significantly worse disease-free survival (DFS), while increased NLR correlated with worse OS and DFS [Citation29]. Lu and colleagues performed a retrospective cohort study on 668 patients with localized prostate cancer who underwent radical prostatectomy and found NLR did not predict cancer specific survival, metastasis free survival, biochemical DFS, OS [Citation30]. Additionally, there was no correlation between Gleason score or lymph node metastasis in this study. A 2018 meta-analysis by Guo and colleagues studied the prognostic roles of NLR and PLR in both localized and advanced PCa patients [Citation31]. This group analyzed 18 studies between 2013 and 2017. The researchers found that NLR and PLR were predictive of poorer OS, as well as worse prostate cancer free survival and biochemical recurrence free survival. Importantly, only 5 of the 18 studies included in the meta-analysis studied localized disease; and specific to the focus on this paper, none of these studies focused on the utility of NLR and PLR in active surveillance counseling. Guan and colleagues recently published a meta-analysis of 3144 patients with mCRPC among 15 cohort studies to evaluate the use of NLR and PLR for prognostic data [Citation32]. Their research suggests that NLR and PLR are promising prognostic markers for mCRPC patients and they support the use of pretreatment NLR and PLR testing to aid with treatment. The combination of these two meta-analyses appear to support the use of NLR and PLR for patients regarding overall and cancer-free survival rates and treatment protocols for mCRPC, respectively. However, they do not address the specific aim of this paper, which is to assess the utility of these hematologic parameters for AS patients who inherently have low-risk, low-volume disease and desire counseling on when – if ever – they should pursue definitive treatment for their diagnosis.
Our prospective study offers value to the current understanding of these hematologic parameters and their use in a large African-American (AA) cohort choosing AS as their first line of treatment. This study validates these findings in a more diverse patient population yet seen in the published literature. Additionally, as a prospective study, rather than a retrospective analysis of patients following radical prostatectomy, it remains more applicable to patients currently being surveilled. Given the known differences in prostate cancer outcomes among AA men, further evaluation of the utility of NLR and PLR among different racial subsets may be of future interest to researchers.
There are several limitations to this study, including the limited follow up. While two and a half years is an extended period, perhaps the insidious nature of PCa may prevent us from fully identifying the role of these ratios in this study population. Additionally, many of the previously published studies correlated NLR and PLR with OS and DFS. Since we have no patients in this prospective study with prostate cancer related mortality, we are unable to make an assessment about these outcomes currently.
Conclusions
NLR and PLR have provided important diagnostic and prognostic information in other malignancies, but they have a limited role in determining the likelihood of pathologic upgrading in patients with localized PCa on AS. Continued inquiry into the differences between localized and metastatic PCa cellular signaling pathways may further elucidate the utility of these hematologic parameters in the treatment of prostate cancer.
Ethics approval
All the procedures were examined and approved by the Joint committee of the Southeast Louisiana Veterans Healthcare System and were in accordance with the ethical standards of the Committee for Human Experimentation, with the Helsinki Declaration of 1975 (revised in Tokyo 2004) and the Committee on Publication Ethics guidelines. All pathological, clinical, or personal data were anonymized and separated from any personal identifiers.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Mahal BA, Butler S, Franco I, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA. 2019;321(7):704–706.
- Catalona WJ. History of the discovery and clinical translation of prostate-specific antigen. Asian J Urol. 2014;1(1):12–14.
- Huang SP, Bao BY, Wu MT, et al. Significant associations of prostate-specific antigen nadir and time to prostate-specific antigen nadir with survival in prostate cancer patients treated with androgen-deprivation therapy. Aging Male. 2012;15(1):34–41. Mar
- Welch HG, Albertsen PC. Reconsidering prostate cancer mortality – the future of PSA screening. N Engl J Med. 2020; 382(16):1557–1563.
- Porpiglia F, Manfredi M, Mele F, et al. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: results from a randomized prospective study in biopsy-naive patients with suspected prostate cancer. Eur Urol. 2017;72(2):282–288. Aug
- Ohwaki K, Endo F, Shimbo M, et al. Comorbidities as predictors of incidental prostate cancer after Holmium laser enucleation of the prostate: diabetes and high-risk cancer. Aging Male. 2017;20(4):257–260.
- Yu JJ, Yan T. Effect of mtDNA mutation on tumor malignant degree in patients with prostate cancer. Aging Male. 2010;13(3):159–165. Sep
- Torrealba N, Rodriguez-Berriguete G, Fraile B, et al. PI3K pathway and Bcl-2 family. Clinicopathological features in prostate cancer. Aging Male. 2018;21(3):211–222. Sep
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641.
- Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124.
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212.
- Spicer JD, McDonald B, Cools-Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72(16):3919–3927.
- Cheng J, Ye H, Liu Z, et al. Platelet-derived growth factor-BB accelerates prostate cancer growth by promoting the proliferation of mesenchymal stem cells. J Cell Biochem. 2013;114(7):1510–1518.
- King Thomas J, Mir H, Kapur N, et al. Racial differences in immunological landscape modifiers contributing to disparity in prostate cancer. Cancers (Basel). 2019;11(12):1857.
- Latini DM, CaPSURE™ Investigators, Elkin EP, Cooperberg MR, et al. Differences in clinical characteristics and disease-free survival for Latino, African American, and non-Latino white men with localized prostate cancer: data from CaPSURE. Cancer. 2006;106(4):789–795.
- Huang SP, Huang CY, Liu CC, et al. Clinical outcome of Taiwanese men with clinically localized prostate cancer post-radical prostatectomy: a comparison with other ethnic groups. Aging Male. 2010;13(1):10–17.
- Zhang JY, Ge P, Zhang PY, et al. Role of neutrophil to lymphocyte ratio or platelet to lymphocyte ratio in prediction of bone metastasis of prostate cancer. Clin Lab. 2019;65(5):853–858.
- Shu K, Zheng Y, Chen J, et al. Prognostic value of selected preoperative inflammation-based scores in patients with high-risk localized prostate cancer who underwent radical prostatectomy. OTT. 2018;11:4551–4558.
- Kwon YS, Han CS, Yu JW, et al. Neutrophil and lymphocyte counts as clinical markers for stratifying low-risk prostate cancer. Clin Genitourin Cancer. 2016;14(1):e1–8. Feb
- Vukovic M, Kavaric P, Magdelinic A, et al. Perineural invasion on biopsy specimen as predictor of tumor progression in aging male treated with radical prostatectomy. Could we use it for pre-surgical screening? Aging Male. 2019;7:1–6.
- Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62(6):976–983. Dec
- Herlemann A, Buchner A, Kretschmer A, et al. Postoperative upgrading of prostate cancer in men >/=75 years: a propensity score-matched analysis. World J Urol. 2017;35(10):1517–1524. Oct
- Jia J, Zheng X, Chen Y, et al. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumor Biol. 2015;36(12):9319–9325.
- Elyasinia F, Keramati MR, Ahmadi F, et al. Neutrophil-lymphocyte ratio in different stages of breast cancer. Acta Med Iran. 2017;55(4):228–232.
- Peng H, Luo X. Prognostic significance of elevated pretreatment systemic inflammatory markers for patients with prostate cancer: a meta-analysis. Cancer Cell Int. 2019;19(1):70.
- Tang H, Li B, Zhang A, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in colorectal liver metastasis: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0159447.
- Ferro M, Musi G, Serino A, et al. Neutrophil, platelets, and eosinophil to lymphocyte ratios predict gleason score upgrading in low-risk prostate cancer patients. Urol Int. 2019;102(1):43–50.
- Gokce MI, Tangal S, Hamidi N, et al. Role of neutrophil-to-lymphocyte ratio in prediction of Gleason score upgrading and disease upstaging in low-risk prostate cancer patients eligible for active surveillance. CUAJ. 2016;10(11-12):383–E387.
- Sun Z, Ju Y, Han F, et al. Clinical implications of pretreatment inflammatory biomarkers as independent prognostic indicators in prostate cancer. J Clin Lab Anal. 2018;32(3):e22277.
- Lu Y, Huang HH, Lau W. Evaluation of neutrophil-to-lymphocyte ratio as a prognostic indicator in a Singapore cohort of patients with clinically localized prostate cancer treated with prostatectomy. World J Urol. 2020;38(1):103–109.
- Guo J, Fang J, Huang X, et al. Prognostic role of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in prostate cancer: a meta-analysis of results from multivariate analysis. Int J Surg. 2018;60:216–223.
- Guan Y, Xiong H, Feng Y, et al. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis. 2020;23(2):220–231.
