?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
We evaluated long-term effects of testosterone undecanoate on glycemic control, metabolic syndrome, vascular function and morphology in obese men with functional hypogonadism (FH) and type 2 diabetes (T2D) in a 2-year prospective clinical trial.
Methods
A total of 55 participants were enrolled in this study; group P (n = 27) received placebo during first and testosterone therapy (TTh) during second year, group T (n = 28) received TTh both years. We pooled results after 1 year of TTh to obtain more statistical power. Results for group T after 2 years of TTh are also presented. We evaluated wide assortment of biochemical (fasting plasma glucose—FPG, glycated hemoglobin—HbA1c and lipid profile), hormonal, vascular (flow-mediated dilatation—FMD and intima-media thickness—IMT), anthropometrical and derived parameters (BMI, HOMA-IR, non-HDL cholesterol, bioavailable and calculated free testosterone). Quality of life was assessed using Aging Males’ Symptoms (AMS) questionnaire.
Results
FPG, HbA1c, HOMA-IR and IMT decreased, FMD increased, lipid profile and AMS sexual sub-score improved, and testosterone levels fully normalized after 2 years of TTh.
Conclusions
Two-year of TTh resulted in normalized serum testosterone levels, improved glycemia, endothelial function, lipids and insulin sensitivity, and quelled the symptoms of hypogonadism, potentially reducing cardiovascular risk in obese men with FH and T2D.
Introduction
More than one-third of men with obesity and either type 2 diabetes (T2D) or metabolic syndrome (MetS) exhibit lowered testosterone levels [Citation1–5]. Functional hypogonadism (FH) is caused by conditions that suppress gonadotropin and testosterone secretion and consequently lower their concentrations, but these conditions are potentially reversible with treatment of the underlying etiology [Citation6,Citation7]. Obesity is considered to be the most frequent cause of FH [Citation8–11]. FH negatively affects glycemic control, exacerbates early cardio-vascular disease, reduces lean body mass, accelerates the accumulation of visceral fat and leads to obesity [Citation9,Citation12–15].
The relationship between FH, MetS, obesity and T2D is complex, multi-directional and involves different mechanisms [Citation9,Citation10,Citation16]. Central component of these connections is inverse association of testosterone and insulin resistance (IR) [Citation17–19]. Improvement of systemic IR by antidiabetic drugs leads to a modest increase in testosterone in men with T2D, but without restoration of normal testosterone levels [Citation20].
Considerable evidence exists from a number of studies that testosterone therapy (TTh) ameliorates components of MetS, improves lipid profile, lowers fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) levels, improves insulin sensitivity, attenuates inflammation, decreases systolic and diastolic pressures in males with T2D [Citation21–26]. Furthermore, long-term TTh completely prevents prediabetes progression to T2D in men with hypogonadism and improves glycemia, lipid profile, and quality of life assessed by Aging Males’ Symptoms (AMS) questionnaire [Citation27].
Clinical evidence suggests that at population level, low testosterone level is a risk factor for cardiovascular events [Citation12,Citation28]. Long-term impact of TTh on vascular function and morphology in obese men with FH and T2D is still unclear. FH has been implicated in the pathogenesis of atherosclerosis and the susceptibility of the myocardium to ischemia, through modulating inflammation as well as vascular endothelial function [Citation29–31]. Endothelial dysfunction is an early functional marker of vascular disease which leads to a change in vascular tone, hypertension and formation of atherogenic plaques [Citation32]. Increased intima-media thickness (IMT) is a feature of atherosclerosis which is an early and potentially reversible morphological marker [Citation33]. Various studies have shown an inverse relationship between testosterone and IMT [Citation34–36]. Both endothelial function and IMT offer prognostic information about the risk of cardiovascular events [Citation37].
The objective of this two-part study was to evaluate effects of TTh on glycemic control, parameters of MetS and vascular function and morphology in a very high-risk population of obese male patients with confirmed symptomatic FH and T2D. The first part of the study focused on effects of TTh compared to placebo, and the second part on long-term (2-year) effects of TTh on those same parameters. We present long-term (2-year) results for study group T (n = 28) and supplement 1-year TTh data with results from the P group, which has been switched to TTh during the second part of the study (groups P and T; n = 55).
Main outcome measures were changes in testosterone levels, glycemic control, parameters of MetS, endothelial function of brachial artery and morphology of carotid arteries. Other outcome measures were assessments of symptoms of hypogonadism and sexual function.
Subjects and methods
Study design
Study on Effects of TTh in Hypogonadal obese type 2 diabetic male patients (SETH2 study) was a two-part single-center prospective observational clinical study (first year double-blind, randomized, placebo-controlled trial; second year open-label follow-up) conducted from January 2014 to March 2018 at General Hospital Celje (Slovenia). In the first part of the study, participants were randomized into groups T and P, and received intramuscular testosterone undecanoate (TU) or matching placebo for 1 year. Participants from P group were switched to TU in the second year (having received placebo during first year of the study) while participants from T group continued receiving TU for a total of 2 years of TTh. Study was approved by the National Medical Ethic Committee (54/04/12) and was conducted in accordance with the Declaration of Helsinki and with all local applicable laws and regulations. Written informed consent was obtained from all study subjects prior to their participation in the study. The study has been registered at ClinicalTrials.gov (identifier: NCT03792321).
Study population
A total of 55 obese Caucasian male patients with confirmed symptomatic FH, aged 40–70 years, with T2D, were recruited to participate in SETH2 trial. FH was diagnosed as a biochemical deficiency of circulating testosterone levels (total testosterone [TT] below 11 nmol/L and free testosterone below 220 pmol/L) on at least two separate morning measurements after an overnight fast in addition to exhibiting at least two symptoms of sexual dysfunction (less frequent morning erections, erectile dysfunction and decreased libido) [Citation6,Citation38,Citation39].
Inclusion criteria for the SETH2 trial were: male, confirmed FH, age > 35 years, BMI ≥ 30 kg/m2, T2D treated exclusively with non-insulin antidiabetic medications (metformin and sulfonylureas). Participants did not use concomitant medications that can reduce weight, BMI and waist circumference.
Exclusion criteria were: previously treated hypogonadism, history of current prostate or breast cancer, severe benign prostatic hyperplasia or prostate specific antigen (PSA) level > 4.0 μg/L, severe heart failure, acute coronary event or procedure during the 6 months prior to the study, chronic obstructive lung disease, severe obstructive sleep apnea with apnea–hypopnea index > 30/h, and active infection.
Inclusion and exclusion criteria were reviewed at screening visit where patients were asked about symptoms and signs suggestive of hypogonadism in accordance with the clinical practice guidelines [Citation6,Citation39], underwent clinical history and physical examination. We performed baseline clinical examination, anthropometric measurements and took blood samples for biochemical and hormonal tests. Data were collected at the Diabetic outpatient clinic at General Hospital Celje (Slovenia).
Study protocol
Subjects were randomly assigned in a concealed 1:1 allocation to either group T or P. An independent statistician generated the randomization sequence. Trial participants and trial investigators were blinded to treatment allocation for the first phase of the study. Group P received placebo and group T received testosterone in the form of testosterone undecanoate (TU, Nebido® 1000 mg; Bayer AG) during the first year (first part) of the study employing the following protocol: first injection of TU/placebo was administered at the first visit (baseline), second injection 6 weeks later (second visit), and each subsequent injection 10 weeks after the previous injection.
All study participants received TU during the second year of the study (second part) since placebo control was limited to 1 year due to ethical and medical concerns over withholding TTh from hypogonadal patients.
Safety parameters (complete blood count, PSA, markers of hepatic and renal functions) were performed at 0, 3, 6, 12, 15, 18 and 24 months. All patients were instructed to report any side effects during the treatment. Patients were not given any advice or instructions regarding dietary or other lifestyle changes prior to and during the study. Use of non-insulin medications was permitted and patients continued on the same (previously prescribed) anti-diabetic medication throughout the course of the study without dose adjustments.
Methods
All patients underwent clinical, anthropometric, biochemical and hormonal assessment along with vascular measurements at baseline, after 1 year and after second year.
Assessment of biochemical, hormonal and metabolic parameters
Fasting blood samples were taken between 7:00 am and 11:00 am to measure serum TT, estradiol, sex hormone binding globulin (SHBG), albumin, luteinizing hormone, follicle-stimulating hormone, FPG, insulin, glycated hemoglobin A1c (HbA1c), lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides), PSA, routine blood tests (complete blood count, electrolytes, urea, creatinine, and liver tests). Anthropometric measurements taken were body weight, height, and waist circumference (WC), along with systolic and diastolic blood pressure. Waist circumference was measured on the horizontal plane between the iliac crest and costal margin of the lower rib; the measurement was taken at the end of expiration.
We calculated the following parameters: BMI, homeostasis model assessment of insulin resistance (HOMA-IR) index, calculated free testosterone (cFT), bioavailable testosterone (BT), and non-HDL cholesterol.
BMI was calculated as the weight in kilograms divided by square of height in meters.
HOMA-IR index was calculated as:
cFT and BT levels were calculated using SHBG, serum albumin and TT level data using Vermeulen’s formulae [Citation40].
Non-HDL cholesterol, which represents the cholesterol content of all proatherogenic, apo-B-containing lipoproteins [Citation41], was calculated as:
Assessment of symptoms and signs of FH
The AMS scale is a standardized questionnaire according to psychometric norms, designed in order to determine symptoms of aging and their severity over the time. The questionnaire, composed of 17 questions each scoring from 1 to 5, identifies three different categories of symptoms: psychological, somatic and sexual. The total AMS score may vary from 17 to 85, and distinguishes five grades of severity, ranging from no/little complaints to severe complaints [Citation42]. AMS questionnaire responses were collected and analyzed before (baseline) and at the end of the study (after 2 years). Sexual function was assessed by questions 15 (sexual ability), 16 (morning erections) and 17 (libido) of AMS sexual function sub-scores.
Assessment of endothelial function
Endothelial function was examined with noninvasive assessment by brachial artery flow-mediated dilatation (FMD) and endothelium-independent nitroglycerine-mediated dilatation (NMD) at the start, after 1 year and after 2 years of the trial [Citation43]. After scanning the baseline artery diameter, the sphygmomanometer cuff was inflated to 50 mmHg above systolic blood pressure and kept for 5 min. Brachial artery diameter was measured again during reactive hyperemia 1 min after cuff deflation. FMD (%) was defined as the percentage change of the artery diameter following reactive hyperemia relative to the baseline diameter.
NMD was provoked by sublingual administration of 400 mg of glycerol-trinitrate, which acts as a nitric oxide donor. NMD (%) was expressed as the percentage change in the diameter after glycerol-trinitrate administration relative to the baseline scan.
Ultrasonography of carotid arteries
All subjects underwent ultrasonography for evaluation of IMT and plaques at the beginning, after 1 year and after 2 years of the trial. Ultrasound (US) examination of the carotid arteries was performed using the General Electric Logiq S7 Expert/Pro ultrasonic device, with 9 L-D probe, frequency band 3.1–10 MHz, in B mode. IMT was measured on three sections of both carotid arteries: communis, internal and bulbus. At each investigated section, IMT measurements were taken three times and the average value for each section was subsequently calculated.
Outcomes
Main outcome measures were assessment of testosterone levels, glycemic control (HbA1c and FPG), lipids, insulin resistance (HOMA-IR), visceral obesity (assessed via WC), blood pressure, endothelial function of brachial artery (assessed via FMD), and morphology of carotid arteries (via IMT). Other outcome measures were assessment of symptoms of hypogonadism by AMS questionnaire.
Statistical analysis
Baseline values reported in this article and in and were established at the point of introduction of TTh in the respective study group (at month 0 for group T participants and at month 12 for group P participants). Pooled P and T group data on effects of TTh on obese males with T2D and FH (after 1 year of receiving TTh; ) and long-term 2-year group T results (after 2 years of TTh; ) were compared to the baseline values.
Shapiro–Wilk test was used to ascertain whether observed changes in data were normally distributed. Values are reported as mean ± SD when changes from the baseline are normally distributed and as median (interquartile range) when distribution sufficiently deviates from normal. Paired samples t-test was employed when change data was normally distributed and Wilcoxon signed ranks test was used for non-normally distributed data.
Extreme outliers were removed and t-tests were re-run when possible to ensure normality of data distribution, with both t-test results (with and without outliers) reported in such occasions—first result set including all outliers is reported within the table body, while the second result set excluding the outliers reported is in the respective footnote underneath the table.
Both 95% CI and p values are reported for t-tests. Only p values are reported in cases where Wilcoxon signed ranks test was employed. Significance level (α) of 0.05 was used. SPSS Statistics 22.0 (IBM Corporation, NY, USA) was used for statistical analysis.
Results
Participants
Baseline demographic, anthropometrical and laboratory parameters of the study population are provided in .
Table 1. Study population characteristics. Values reported as mean ± SD or as count (percentage).
Changes in outcome measures
Results of the first year placebo-controlled portion of the trial showcasing groups P (placebo) and T (TTh) have been published previously [Citation44]. To summarize, we observed reductions of HOMA-IR, FPG levels, HbA1c and total cholesterol levels after 1 year of TTh in group T, as opposed to the group P. FMD improved in group T, IMT decreased in both groups after 1 year, but IMT decrease in group T was twice that of group P. Statistically significant decrease of BMI and WC was observed in both groups.
Pooled results after 1 year of TTh
We pooled results obtained after 1 year of TTh (after first year of the trial for T group and after second year of the trial for P group; n = 55) to obtain more power to detect changes in outcomes in comparison to paired samples results after 1 year of TTh which have been presented in the initial article for group T (n = 28). Complete pooled results are shown in .
Glycemic control
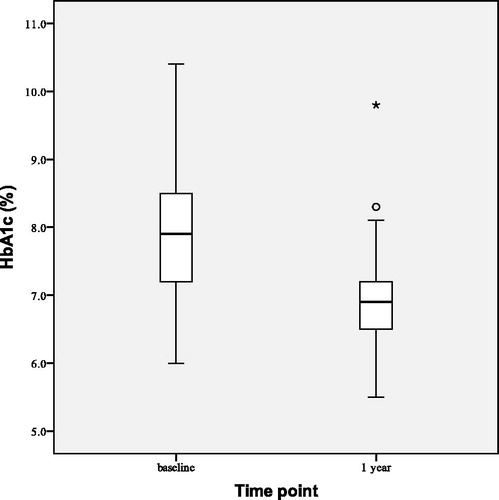
FPG decreased by 1.21 ± 1.07 mmol/L (p < 0.001) and HbA1c decreased by 0.96 ± 0.73% points (p < 0.001) in our 55 study participants after 1 year of TTh (). Only four group P patients (out of 27) and three group T patients (out of 28) has HbA1c < 7.00% before commencing TTh; after 1 year of TTh 19 group P patients and 11 group T patients had HbA1c < 7.00%.
Metabolic parameters
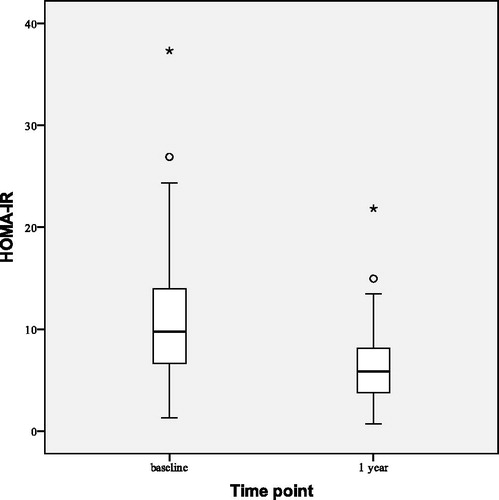
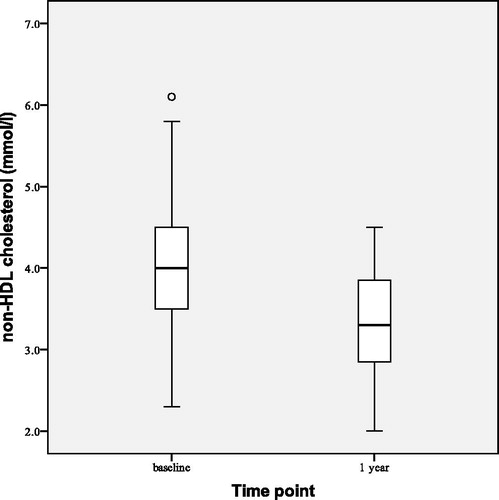
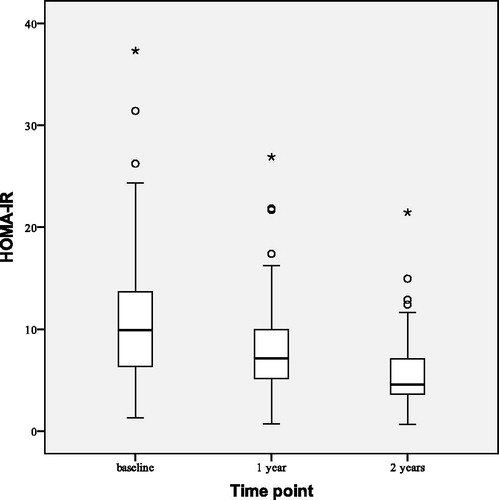
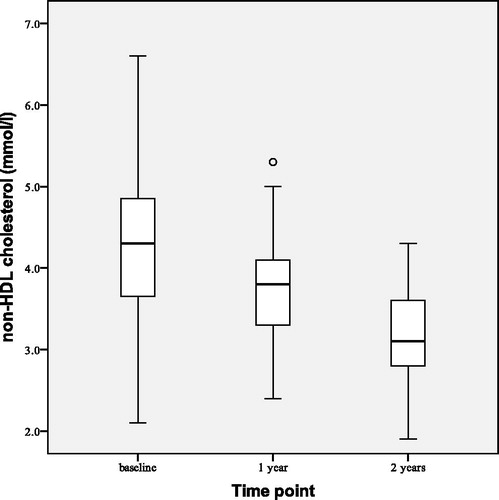
Statistically significant median reductions of BMI by 0.70 (0.00–1.30) kg/m2 (p < 0.001) and WC by 1.50 (0.70–2.90) cm (p < 0.001) were observed after 1 year of TTh (). HOMA-IR index decreased () by 4.26 ± 3.65 (p < 0.001). We also observed reductions of total cholesterol by 0.69 ± 0.57 mmol/L (p < 0.001), LDL cholesterol by 0.28 ± 0.58 mmol/L (p = 0.001) and triglyceride level by 0.51 ± 1.17 mmol/L (p = 0.002). Non-HDL cholesterol decreased () by 0.74 ± 0.62 mmol/L (p < 0.001). No statistically significant changes from the baseline were observed in HDL cholesterol level, systolic blood pressure or diastolic blood pressure.
Table 2. Effects of testosterone treatment on parameters of metabolic syndrome after 12 months of testosterone replacement therapy—pooled results for both groups P and T (n = 55).
Vascular function and morphology
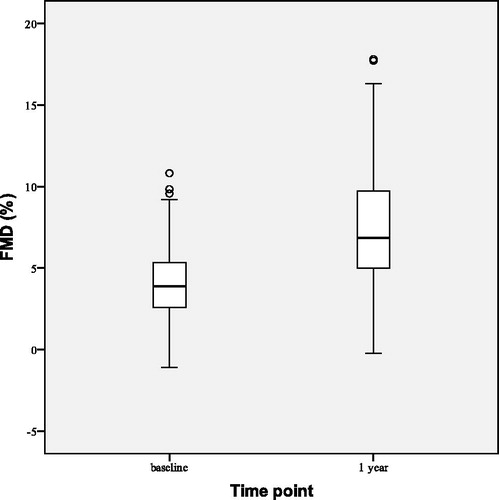
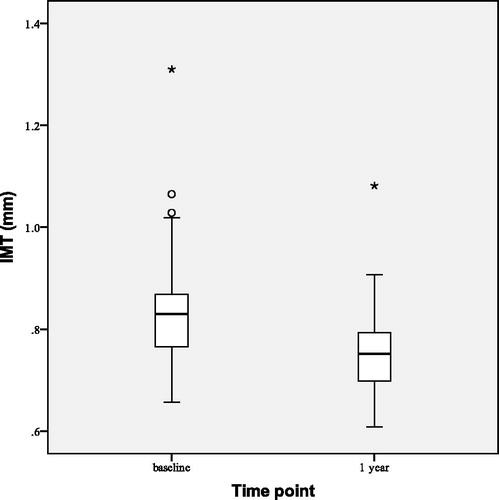
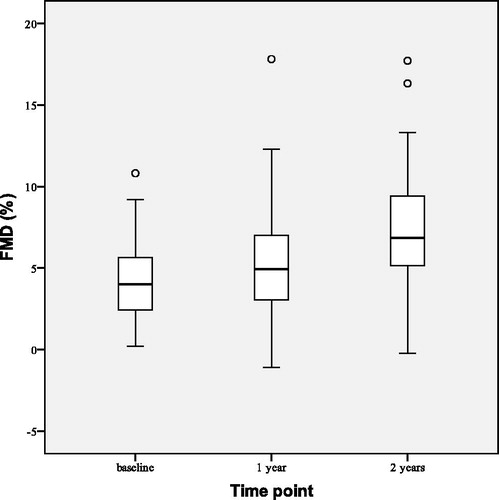
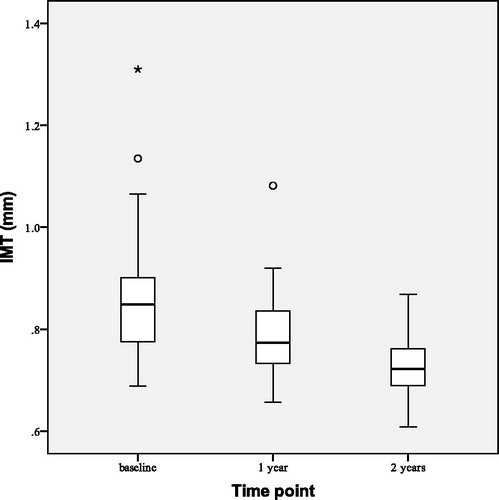
FMD improved () by 3.12 ± 4.97% points (p < 0.001) and FMD/NMD ratio increased from 0.29 ± 0.20 to 0.46 ± 0.24 (p < 0.001). IMT decreased slightly (), but at a statistically significant level, by 0.08 ± 0.06 mm (p < 0.001).
Testosterone levels
TTh resulted in increase of TT by 8.96 ± 3.04 nmol/L (p < 0.001), calculated BT by 5.38 ± 2.06 nmol/L (p < 0.001) and calculated FT by 230.75 ± 93.12 pmol/L (p < 0.001). There was no statistically significant change in SHBG levels after 1 year of TTh.
Results after 2 years of TTh
Two-year results for group T (n = 28) participants are presented as these patients were receiving TTh uninterrupted over a course of 2 full years. contains exhaustive list of outcomes following 2 years of TTh.
Table 3. Effects of testosterone treatment on parameters of metabolic syndrome after 24 months of therapy—results for group T (n = 28).
Glycemic control
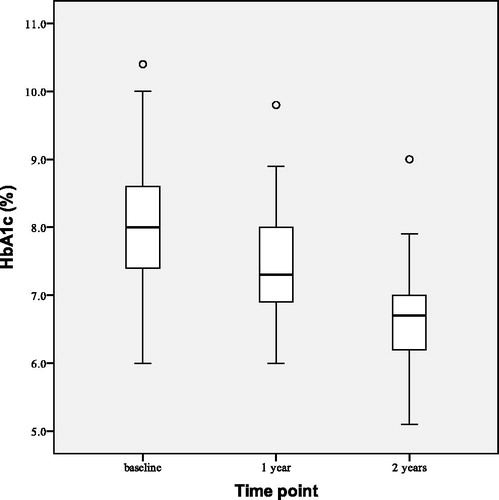
depicts observed changes in HbA1c at the beginning (baseline), after 1 and 2 years of the study. After 2 years of TTh both HbA1c and FPG were reduced. HbA1c decreased by 1.51 ± 0.90% points (p < 0.001), and FPG by 1.83 ± 0.20 mmol/L (p < 0.001). The number of group T patients with HbA1c < 7.00% rose from baseline value of 3–20 (out of 28) after 2 years of TTh.
Metabolic parameters
shows observed changes in HOMA-IR at the beginning (baseline), after 1 and 2 years of the study. After 2 years of TTh HOMA-IR index decreased by 5.94 ± 4.31 (p < 0.001), BMI decreased by 1.59 ± 1.44 kg/m2 (p < 0.001) and WC decreased by 3.51 ± 2.73 cm (p < 0.001). All lipid profile parameters improved at statistically significant levels: total cholesterol decreased by 0.97 ± 0.59 mmol/L (p < 0.001), LDL cholesterol by 0.40 ± 0.65 mmol/L (p = 0.003) and triglyceride level by 0.99 ± 1.23 mmol/L (p < 0.001) while HDL cholesterol increased by 0.15 ± 0.21 mmol/L (p = 0.001). This led to decrease in non-HDL cholesterol () by 1.12 ± 0.67 mmol/L (p < 0.001). No statistically significant changes have been observed in systolic and diastolic blood pressure.
Vascular function and morphology
After 2 years of TTh FMD increased () by 2.46 ± 3.97% points (p = 0.003) from the baseline and FMD/NMD ratio increased from baseline value of 0.31 ± 0.21 to 0.44 ± 0.21 (p = 0.023). Median IMT decreased () by 0.14 (0.10–0.17) mm (p < 0.001).
Testosterone levels
As expected, TTh resulted in significant increase in testosterone levels: TT increased by 16.26 ± 4.89 nmol/L (p < 0.001) from the baseline, calculated BT increased by 10.19 ± 3.42 nmol/L (p < 0.001) and calculated FT by 438.50 ± 146.87 pmol/L (p < 0.001). No statistically significant changes have been observed in SHBG.
Symptoms of FH
AMS questionnaire sexual sub-score (consisting of questions 15–17) decreased from median of 9.00 (8.00–11.75) to 8.00 (6.00–9.00) (p = 0.005), indicating an improvement, while the total AMS score did not change at a statistically significant level after 2 years of TTh (p = 0.060).
Safety parameters
TTh led to expected increases in hemoglobin and hematocrit concentrations, which remained within normal reference range during the entire 2-year observation period.
Adverse events
No adverse events or side effects of TTh have been observed over the 2-year course of this trial.
Discussion
This is the first long-term (2 year) study with exclusive focus on obese men with FH and T2D, which evaluated the effects of TTh on vascular function and morphology along with wide assortment of metabolic and glycemic parameters. We previously showed that 1-year of TTh in these high risk patients resulted in reductions of HOMA-IR, FPG levels, HbA1c and total cholesterol levels and improvement of FMD of brachial arteries and IMT of carotid arteries. Two-year therapy with TTh in obese men with FH and T2D further improved glycemic control, IR, dyslipidemia, and endothelial function in addition to restoring serum testosterone levels well into normal range.
Our findings demonstrate that TTh led to substantial improvements in glycemic parameters in obese men with FH and T2D. HbA1c decreased from 7.89% to 6.93% after 1 year of TTh in pooled study population. Reduction from 8.12% to 6.60% in group T after 2 years of TTh was even more pronounced and followed previous decrease to 7.18% after first year of TTh [Citation44]. This was accompanied by reductions in FPG from 9.77 to 8.57 mmol/L in pooled study population after 1 year of TTh and from 10.06 to 8.23 mmol/L in group T after 2 years of TTh, following decrease to 8.83 mmol/L after first year of TTh [Citation44]. Elevated HbA1c is both a reliable biomarker for the diagnosis and prognosis of T2D and a significant risk factor for cardiovascular diseases and stroke in subjects with T2D. Clinical implications of reducing the HbA1c level are improvement in glycemic control and lowering of mortality by prevention of both microvascular and macrovascular complications of T2D.
Several studies have demonstrated that TTh is associated with improvement in metabolic parameters. Reported effects of TTh on HbA1c and FPG vary [Citation22,Citation45,Citation46].
We observed significant and sustained reduction in HOMA-IR after 2 years of TTh in obese men with FH and T2D. Improved insulin sensitivity definitely impacted on glycemic control. Significant changes in HbA1c and insulin sensitivity reflect the importance of TTh in regulation of hyperglycemia in men with FH and T2D. Some studies in non-obese men did not show a change in insulin sensitivity following TTh [Citation21], which implies that testosterone-induced increase in insulin sensitivity occurs only in obese and insulin-resistant men [Citation47].
Our results also showed modest, but statistically significant reduction in BMI, WC, and body weight after 2 years of TTh. It is worth noting that BMI in group T patients decreased by 0.80 kg/m2 both after first and again after second year of TTh; likewise, reduction of WC we observed after second year of TTh was comparable in magnitude to that after first year of TTh, emphasizing long-term effects of testosterone on certain metabolic parameters. TTh is known to improve IR through reduction of visceral obesity; androgen receptors are both larger and found in greater numbers in visceral adipocytes than in subcutaneous adipose tissue [Citation48]. As demonstrated by several studies TTh reduces body weight, WC, BMI and fat mass in both obese and non-obese men [Citation49]. Conversely, testosterone levels increased in men who lost weight and visceral mass by dieting, physical activity or bariatric surgery [Citation50]. Prospective studies of men with FH who were receiving TU showed 5% decrease in body weight in the first year of TTh and greater than 13% decrease after 5 years [Citation51]. These effects are most notable in patients with high BMI [Citation52]. Testosterone increases lean body mass, an effect that is superior to other drugs (anti-obesity or antidiabetic medications) either alone or in combination with behavioral and lifestyle modifications [Citation53].
We observed a statistically significant decrease in total cholesterol, LDL cholesterol, non-HDL cholesterol and triglyceride level, while HDL cholesterol level has increased after 2 years of TTh in group T patients, following a smaller, but statistically significant decrease of total cholesterol, LDL cholesterol and triglyceride levels after 1 year of TTh in pooled study population results. Non-HDL is superior predictor of coronary heart disease than LDL-cholesterol level alone in patients with T2D; several medical organizations and clinical guidelines recommend that non-HDL be the primary target for reducing CVD risk in men with T2D [Citation54]. This improvement in atherogenic dyslipidemia contributes to the improvement of endothelial function in our study, reducing cardiovascular mortality in obese men with T2D.
We showed that endothelial function (assessed by FMD) in obese men with FH and T2D has improved marginally from 1-year results, but at statistically significant level, after 2 years of TTh. Significant increase in FMD/NMD ratio indicates that brachial artery dilation improved due to amelioration of endothelial dysfunction rather than the improvement of vascular smooth muscle function of the arterial wall. Other studies investigating the relationship between testosterone levels and FMD have shown varying results [Citation25,Citation55,Citation56]. The improvement of endothelial function following TTh could be due to direct effects of testosterone through different means: via androgen receptors, which are present on the vessel wall in endothelial and vascular smooth muscle cells; by moderating regulation of vascular tone by increasing vasodilatation in coronary arteries; by reducing the levels of endothelin-1, a major vasoconstrictor, the level of which is increased in men with FH. TTh has been shown to reduce the IMT regardless of BMI, while some studies have investigated effect of TTh on IMT with varying results [Citation37,Citation57]. Testosterone is known to act on vasculature by influencing both systemic inflammation and leucocyte activation to reduce localized atheroma development of blood vessels [Citation58]. Our data shows that IMT has improved at statistically significant levels following TTh.
Low testosterone levels in men are associated with high levels of inflammatory markers in various clinical conditions such as obesity [Citation59], MetS [Citation60], carotid atherosclerosis [Citation61], T2D [Citation62] and hypogonadism [Citation63]. The effect of TTh on secretion of inflammatory cytokine levels in men have been investigated by a number of studies [Citation47,Citation64,Citation65]. Testosterone has been shown to inhibit pro-inflammatory cytokines TNFα, INFβ and IL6 released from cultured peripheral monocytes isolated from androgen-deficient men with T2D [Citation66]. Additionally, TTh has been reported significantly reduce TNFα and elevate circulating anti-inflammatory IL10 in men with hypogonadism and cardiovascular disease [Citation67]. Following 1 year of TTh with TU, CRP decreased significantly in men with hypogonadism [Citation68]. Some studies however failed to show statistically significant relationship between TTh and anti-inflammatory effects of testosterone [Citation69]. The causal relationship between testosterone and inflammation can only be confirmed in a longitudinal study [Citation70].
No statistically significant changes have been observed in either systolic or diastolic blood pressure in our patients following TTh which is in accordance with findings of other clinical trials and some meta-analyses [Citation71], but in contrast to some very long-term observational studies [Citation51], so the effect of TTh on blood pressure may well be a function of treatment duration.
As expected, TT, cFT and BT concentrations have increased significantly after 1 year of TTh, pushing TT level of 54 of our 55 patients above the 11 nmol/L reference threshold, while the sole exception, a patient with baseline BMI of 41.0 kg/m2 and TT of 7.50 nmol/L reached TT of 10.60 nmol/L (and ultimately 18.40 nmol/L after 2 years of TTh). We observed additional increase of testosterone levels after the second year of TTh. No subject ever reached supraphysiological concentrations of testosterone. Other studies employing TU observed a similar increase in TT over time [Citation46]. SHBG levels did not change at a statistically significant level at any point during the course of the study. Any increase in SHBG (which might have been expected due to decrease of IR) could have been offset by the increase in circulating free testosterone, as number of SHBG binding sites for testosterone was reduced [Citation72].
As a result of normalization of testosterone levels by TTh participants also exhibited improvement of sexual symptoms of FH; according to AMS questionnaire their median sexual sub-score decreased (meaning that their sexual function improved) which impacts quality of life in long-term.
Limitations and strengths
One limitation of this study is that we have not been able to perform objective assessment of changes in body composition (measurement of lean body mass and fat mass using dual-energy X-ray absorptiometry) for better evaluation of the efficacy of observed weight loss and decrease of WC. Another limitation is that duration of placebo-versus-TTh comparison was limited to 1 year due of the ethical and medical concerns over withholding testosterone treatment from male patients with FH for extended periods of time, however this limitation pertains to all placebo-controlled testosterone trials.
Key strength of our study is its exclusive focus on obese males with T2D and FH, a population deemed to be at a very high risk for glycometabolic and cardiometabolic complications; to our knowledge, a first. Further strengths of our study include the extended (2-year) duration of TTh and zero study dropouts over its course of 2 years; study participants were highly motivated by participation in the trial, which—in additional to complete absence of any adverse events that would otherwise result in termination of TTh—undoubtedly contributed to the zero drop-outs record. Furthermore, since intramuscular injections of TU were used, adherence to treatment was 100%.
In conclusion, several cardiovascular risk factors have improved in obese men with FH and T2D after 2 years of TTh, most notably IR, components of atherogenic dyslipidemia and glycemic control, resulting in weight loss, improvement in endothelial function and vascular morphology. Testosterone affects these parameters differently in long-term; some see further notable improvements with continuation of TTh beyond 1 year (BMI, WC, HbA1c, triglycerides, LDL cholesterol, HDL cholesterol and IMT). These findings, together with the therapeutic benefit of testosterone with regard to symptoms of hypogonadism, call for additional, longer-term studies on effects of TTh in obese males with FH and T2D.
Acknowledgements
Bayer AG (Berlin, Germany) provided testosterone and placebo for this study, but had no role in design of the study protocol, data collection, data analysis or writing of this manuscript.
Disclosure statement
The authors declare that there are no conflicts of interest to report.
Additional information
Funding
References
- Dandona P, Dhindsa S, Chandel A, et al. Hypogonadotropic hypogonadism in men with type 2 diabetes. Postgrad Med. 2009;121(3):45–51.
- Dandona P, Dhindsa S, Chaudhuri A, et al. Hypogonadotrophic hypogonadism in Type 2 diabetes, obesity and the metabolic syndrome. Curr Mol Med. 2008;8(8):816–828.
- Zhang J, Li X, Cai Z, et al. Association between testosterone with type 2 diabetes in adult males, a meta-analysis and trial sequential analysis. Aging Male. 2019;1–12. DOI:10.1080/13685538.2018.1557139
- LeRoith D. Diabetes in the aging male. Aging Male. 2005;8(3–4):133–134.
- Dhindsa S, Ghanim H, Batra M, et al. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care. 2018;41(7):1516–1525.
- Bhasin S, Brito JP, Cunningham GR, et al. Testosterone therapy in men with hypogonadism: an Endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1–30.
- Corona G, Rastrelli G, Morelli A, et al. Treatment of functional hypogonadism besides pharmacological substitution. World J Mens Health. 2020;38(3):256.
- Corona G, Rastrelli G, Monami M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168(6):829–843.
- Grossmann M, Ng Tang Fui M, Cheung AS. Late-onset hypogonadism: metabolic impact. Andrologia. 2019. DOI:10.1111/andr.12705
- Carrageta DF, Oliveira PF, Alves MG, et al. Obesity and male hypogonadism: tales of a vicious cycle. Obes Rev. 2019;20(8):1148–1158.
- Fernandez CJ, Chacko EC, Pappachan JM. Male obesity-related secondary hypogonadism—pathophysiology, clinical implications and management. Eur Endocrinol. 2019;15(2):83–90.
- Wang C, Jackson G, Jones TH, et al. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care. 2011;34(7):1669–1675.
- Deng C, Zhang Z, Li H, et al. Analysis of cardiovascular risk factors associated with serum testosterone levels according to the US 2011–2012 National Health and Nutrition Examination Survey. Aging Male. 2019;22(2):121–128.
- Mazur A, Westerman R, Werdecker A, et al. Testosterone and type 2 diabetes in men. Aging Male. 2014;17(1):18–24.
- Ho C-H, Wu C-C, Chen K-C, et al. Erectile dysfunction, loss of libido and low sexual frequency increase the risk of cardiovascular disease in men with low testosterone. Aging Male. 2016;19(2):96–101.
- Dimopoulou C, Goulis DG, Corona G, et al. The complex association between metabolic syndrome and male hypogonadism. Metab Clin Exp. 2018;86:61–68.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33(6):1186–1192.
- Dandona P, Dhindsa S, Chaudhuri A, et al. Hypogonadotrophic hypogonadism in type 2 diabetes. Aging Male. 2008;11(3):107–117.
- Yassin AA, Saad F, Haider A, et al. The role of the urologist in the prevention and early detection of cardiovascular disease. Arab J Urol. 2011;9(1):57–62.
- Kapoor D, Channer KS, Jones TH. Rosiglitazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab Vasc Dis Res. 2008;5(2):135–137.
- Basu R, Man CD, Campioni M, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30(8):1972–1978.
- Heufelder AE, Saad F, Bunck MC, et al. Fifty-two week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30(6):726–733.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19(1):64–69.
- Saad F, Caliber M, Doros G, et al. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23(1):81–92.
- Khripun I, Vorobyev S, Belousov I, et al. Influence of testosterone substitution on glycemic control and endothelial markers in men with newly diagnosed functional hypogonadism and type 2 diabetes mellitus: a randomized controlled trial. Aging Male. 2019;22(4):241–249.
- Haider KS, Haider A, Saad F, et al. Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes Obes Metab. DOI:10.1111/dom.14122
- Yassin A, Haider A, Haider KS, et al. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: eight-year data from a registry study. Dia Care. 2019;42(6):1104–1111.
- Vlachopoulos C, Ioakeimidis N, Miner M, et al. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis. 2014;233(1):278–283.
- Jones TH, Saad F. The effects of testosterone on risk factors for, and the mediators of, the atherosclerotic process. Atherosclerosis. 2009;207(2):318–327.
- Antonopoulos AS, Antoniades C. Mechanisms of testosterone deficiency-related endothelial dysfunction: invited commentary for the Hellenic Journal of Cardiology on: Tsikas et al. “Associations between asymmetric dimethylarginine, nitrite-dependent renal carbonic anhydrase activity and plasma testosterone levels in hypogonadal men”. Hellenic J Cardiol. 2018;59(4):207–208.
- Rezanezhad B, Borgquist R, Willenheimer R, et al. Association between serum levels of testosterone and biomarkers of subclinical atherosclerosis. Aging Male. 2018;21(3):182–186.
- Mudau M, Genis A, Lochner A, et al. Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovasc J Afr. 2012;23(4):222–231.
- Crouse JR, Craven TE, Hagaman AP, et al. Association of coronary disease with segment-specific intimal-medial thickening of the extracranial carotid artery. Circulation. 1995;92(5):1141–1147.
- Muller M, van den Beld AW, Bots ML, et al. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109(17):2074–2079.
- Kwon H, Lee D-G, Kang HC, et al. The relationship between testosterone, metabolic syndrome, and mean carotid intima-media thickness in aging men. Aging Male. 2014;17(4):211–215.
- Demirbag R, Yilmaz R, Ulucay A, et al. The inverse relationship between thoracic aortic intima media thickness and testosterone level. Endocr Res. 2005;31(4):335–344.
- Zitzmann M, Vorona E, Wenk M, et al. Testosterone administration decreases carotid artery intima media thickness as a marker of impaired vascular integrity in middle-aged overweight men. J Mens Health. 2009;6(3):153–243.
- Wu FCW, Tajar A, Beynon JM, et al.; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135.
- Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males. Andrology. 2020:1–18. DOI:10.1111/andr.12770
- Free & Bioavailable Testosterone calculator [Internet]. [cited 2020. Jul 1]. Available from: http://www.issam.ch/freetesto.htm.
- Liu J-R, Liu B-W, Yin F-Z. Change in nonhigh-density lipoprotein cholesterol levels in adults with prediabetes. Medicine (Baltimore). 2017;96:e8461.
- Heinemann LAJ, Saad F, Zimmermann T, et al. The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15.
- Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol. 2000;50(5):397–404.
- Groti K, Žuran I, Antonič B, et al. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male. 2018;21:1–12.
- Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906.
- Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy improves metabolic parameters in hypogonadal men with type 2 diabetes but not in men with coexisting depression: the BLAST study. J Sex Med. 2014;11(3):840–856.
- Dhindsa S, Ghanim H, Batra M, et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Dia Care. 2016;39(1):82–91.
- Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108(3–5):272–280.
- Salman M, Yassin D-J, Shoukfeh H, et al. Early weight loss predicts the reduction of obesity in men with erectile dysfunction and hypogonadism undergoing long-term testosterone replacement therapy. Aging Male. 2017;20(1):45–48.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–1840.
- Francomano D, Bruzziches R, Barbaro G, et al. Effects of testosterone undecanoate replacement and withdrawal on cardio-metabolic, hormonal and body composition outcomes in severely obese hypogonadal men: a pilot study. J Endocrinol Invest. 2014;37(4):401–411.
- Yassin A, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clin Obes. 2013;3(3–4):73–83.
- Hoyos CM, Killick R, Yee BJ, et al. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol. 2012;77(4):599–607.
- Grundy SM, Arai H, Barter P, et al. An International Atherosclerosis Society Position Paper: global recommendations for the management of dyslipidemia-full report. J Clin Lipidol. 2014;8(1):29–60.
- Mazo E, Gamidov S, Sotnikova E. Effects of different treatments on endothelial function in patients with erectile dysfunction and hypogonadism. Ter Arkh. 2008;80:59–63.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7(10):3495–3503.
- Basaria S, Harman S, Travison TG, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. 2015;314(6):570–581.
- Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47–R71.
- Tremellen K, McPhee N, Pearce K. Metabolic endotoxaemia related inflammation is associated with hypogonadism in overweight men. Basic Clin Androl. 2017;27:5.
- Laaksonen DE, Niskanen L, Punnonen K, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149:601–608.
- Soisson V, Brailly-Tabard S, Helmer C, et al. A J-shaped association between plasma testosterone and risk of ischemic arterial event in elderly men: the French 3C cohort study. Maturitas. 2013;75(3):282–288.
- Bhatia V, Chaudhuri A, Tomar R, et al. Low testosterone and high C-reactive protein concentrations predict low hematocrit in type 2 diabetes. Diabetes Care. 2006;29(10):2289–2294.
- Bobjer J, Katrinaki M, Tsatsanis C, et al. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PloS One. 2013;8(4):e61466.
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612.
- Traish AM, Haider A, Doros G, et al. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314–329.
- Corrales JJ, Almeida M, Burgo R, et al. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189(3):595–604.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318.
- Haider A, Gooren LJG, Padungtod P, et al. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes. 2010;118(3):167–171.
- Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602.
- Mohamad N-V, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–140.
- Grossmann M. Testosterone and glucose metabolism in men: current concepts and controversies. J Endocrinol. 2014;220:37–55.
- Goldman AL, Bhasin S, Wu FCW, et al. A reappraisal of Testosterone’s Binding in Circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302–324.