Abstract
Introduction
Prostate cryotherapy is an available treatment option for localized prostate cancer (PC) included on minimal invasive therapies but still under evaluation. We started our cryotherapy program in 2008 for selected patients with localized PC. Our objective is to evaluate the oncologic and functional outcomes of primary cryotherapy in men with clinically localized PC.
Subjects and Methods
We retrospectively evaluated all patients who underwent primary cryotherapy for localized PC treatment at our center between January 2008 and December 2017. In order to downsize prostates between 40 and 60cc neoadjuvant 3-month hormonal therapy was administered. Primary endpoint was biochemical progression-free survival (BPFS) rate as defined by the Phoenix criteria. Secondary endpoints were cancer-specific survival (CSS), overall survival (OS), patient reported functional outcomes and complication rates. Factors influencing de BPFS were evaluated individually using Kaplan–Meyer and Cox regression models and in a multivariate model using Cox regression.
Results
During the mentioned period, a total of 177 men were treated with cryotherapy. With a mean follow-up of 60 months (SD 32.9), the Kaplan–Meier analysis shows an overall BPFS rate was 67%. BPFS by risk group was 70.2%, 70.3% and 50.0% for the low, intermediate and high risk groups, respectively (p = 0.925). Overall time to BR was 93.67 months (SD 2.84, IC95%: 88.10–99.24): 95.91 (SD 3,44), 93.23 (SD 4.81) and 89.77 (SD 6.67) months for the low, intermediate and high risk groups, respectively. In both univariate and multivariate analysis, the only predictor of biochemical progression was de PSA nadir (HR 1.56 IC95%: 1.50–1.63). Continence was fully maintained in 95% of patients after the procedure. Postoperative complications included UTI (17.5%), hematuria (9.6%), perineal hematoma (11%) and postoperative pain (4.5%). No fistulas were reported. 8.5% of patients had acute urinary retention solved conservatively.
Conclusion
Cryotherapy is a safe option for selected patients with localized prostate cancer that provides competitive oncologic outcomes and a low morbidity profile.
Introduction
Prostate cancer (PC) is the second most commonly diagnosed cancer in men and its prevalence is related to aging, increasing per decade, from a prevalence of 5% at <30 years to 59% (48–71%) by age > 79 years [Citation1]. Life expectancy in developed countries continues to increase over the past century, so that the PC patient profile is changing [Citation2]. In localized PC disease, over 10 years life expectancy is considered mandatory for any benefit from local treatment, however, in the USA, only 41% of patients aged > 75 years with intermediate- and high-risk disease receive curative treatment and although many elderly men who are diagnosed with prostate cancer will die from other causes, 71% of all prostate cancer deaths occur in men aged >75 years [Citation3,Citation4].
Radical prostatectomy (RP) and radiation therapy (RT) with or without androgen deprivation therapy (ADT) have been for the last decades the gold standard treatment with curative intent of localized PC, while ADT has classically been the preferred treatment option in elderly patients. However, none of these treatments are exempt of morbidity and toxicities that may adversely affect the elderly patient’s quality of life [Citation5]. In the past years, other modalities have emerged as potential therapeutic options for localized PC, using different energies to perform prostate ablation with a curative intent. All these technologies have been developed as minimally invasive procedures to provide equivalent oncological control, with reduced toxicity and improved functional outcomes [Citation6]. The current technology of cryosurgery, which represents the third generation equipment, include gas-driven miniaturized equipment using pressurized argon for freezing and helium for thawing, use of thermal sensors, urethral warmer and TRUS ice-ball monitoring, that have led to more efficient prostate gland freezing, reducing collateral damage and in consequence reduced morbidity [Citation7]. Over this time, advances in technology and standardization of method have led to a widespread application of the procedure, with corresponding improvements in efficacy and safety. The objective of the study was to analyze our series of prostate cryotherapy with special interest in postoperative results, complication rates and oncologic results performed between January 2008 and December 2017.
Materials and methods
Patient’s characteristics
We retrospectively reviewed clinical charts of patients treated by primary prostate cryoablation for localized PC at our institution between January 2008 and December 2017. All patients were diagnosed with clinically localized prostate adenocarcinoma (T1-2, N0, M0) with a systematic transrectal prostate biopsy. only primary prostate cryoablation were analysed. Indication for cryosurgery at our center are patients with localized PC over 70 years old, or >65 years unfit for radical prostatectomy. Prostate volume should be below or equal to 40 cc. Patients with prostates between 40 and 60 cc require hormonal therapy for 3 months to downsize the prostate volume.
Clinical and pathological data were recorded. Patients were classified as low, medium or high risk according to D’Amico’s classification [Citation8]. Follow-up include clinical assessment and PSA the first and third month after surgery, then every 6 months during the first 3 years and yearly until 10 years after surgery. Biochemical recurrence (BR) was defined according to the Phoenix definition (an elevation of PSA level ≥2 ng/mL above the post-treatment nadir PSA) [Citation9]. Magnetic resonance image (MRI) and or prostate biopsy was indicated in case of local recurrence suspicious. Recurrent disease was defined as any evidence of clinical recurrence (by physical exam, imaging or biopsy) or initiation of salvage treatment or androgen deprivation therapy. Patients underwent biopsy if candidates to salvage local treatment.
Surgical data analyzed was surgical time, number of cryoprobes inserted, number of cycles and temperatures reached. Early post-surgery complications were recorded as well as continence, irritative symptoms and erectile dysfunction (ED). Urinary symptoms were followed within the first year after the procedure. Urinary incontinence (UI) was considered if the patient required the use of pads (≥1/day). Erectile dysfunction was not asses.
Cryoablation principles
The third generation cryoablative system is an argon gas-based system that works via pressurized gas expansion at the tip of the cryoneedle based on the Joule-Thompson effect. Argon and helium gases are used sequentially for rapid freezing and thawing, respectively, this process creates the freeze–thaw cycle. Cryoablation uses controlled freezing reaching temperatures of lower than −40 °C to cause cell death by different pathophysiological mechanisms including direct injury to the cells caused by ice intracellular crystal formation, failure of the microcirculation following thawing, and the induction of apoptosis and necrosis [Citation6,Citation10].
Surgical technique
All cases were carried out following the same surgical protocol, performed by two surgeons (MJR and AF). The technique was performed under general anesthesia. The patient was prepared with antibiotic prophylaxis and cleaning enema. The patient was positioned in dorsal lithotomy, facilitating a good exposure of the perineum and the handling of transrectal transducer (longitudinal biplane probe). In all cases, we used the CryoCare CS® HealthTronics (Inc. Austin, TX, USA), a third-generation cryotherapy equipment that uses argon for the freeze cycle and helium for the heating cycle. Temperature was monitored inside and outside the prostate. The thermal sensors were placed in the apex, external sphincter, and left and right neurovascular bundles. Hydrodistention of the prostatorectal area was done by injecting saline solution with broad-spectrum antibiotic at Denonvilliers’ space such as protection of the rectal wall (Onik Manoeuvre). Control cystoscopy was performed to ensure the indemnity of the urethra, which is protected by using a continuous flow system which circulates saline with methylene blue and keeps adjacent tissues to a temperature around 38 °C. Depending on the prostate anatomy, from 6 to 8 cryoprobe needles of 2.4 mm of diameter were placed approximately 1 centimeter apart, allowing the overlap of adjacent ice balls for optimal whole gland cryablation. Two complete freeze/thaw cycles were performed. Patients were discharged after 24 h, maintaining the bladder catheter for two or three weeks. Anti-inflammatory and oral antibiotics were prescribed for two weeks and alpha blockers for 1 month () [Citation6,Citation11,Citation12].
Outcomes and statistics
The primary endpoint of the study was the biochemical progression-free survival (BPFS). Secondary outcomes were cancer-specific survival (CSS), overall survival (OS), patient reported functional outcomes and complication rates. Factors influencing de BPFS were evaluated individually using Kaplan–Meyer and Cox regression models and in a multivariate model using Cox regression. IBM® SPSS® v.22.0 (IBM Corp., Armonk, NY, USA) was used for the statistics.
Results
Patient’s demographics and tumor characteristics
During the mentioned period, a total of 177 men underwent primary whole gland cryotherapy for localized PC. Patient’s demographics and tumor characteristics are shown in . Around one third of patients (62 patients) received hormonal treatment for 3 months in order to downsize the prostate. Mean prostate volume after hormonal therapy was 32 cc (SD 9.36). Mean surgical time was 1.55 h (range 1–2.5). Six cryoprobe needles were used in most cases (range 4–8) and two freezing-heating cycles were performed (except in 7 patients who required 3 cycles and one patient 1 cycle). Mean freezing temperature was minus 45.96 °C and mean sphincter temperature was 25.21 °C.
Table 1. Patient’s characteristics.
Postoperative outcomes
Early postoperative complications are shown in , being the most frequent UTI (17.5%), perineal hematoma (11%) and hematuria (9.6%). 5 patients (2.8%) required re-hospitalization for intravenous antibiotic treatment. There were no fistulas. Acute urinary retention was solved conservatively with more days of catheterization, none of them needing further treatment. All complications were Clavien-Dindo classification 1 and 2.
Table 2. Early postoperative complications (%).
Oncologic outcomes
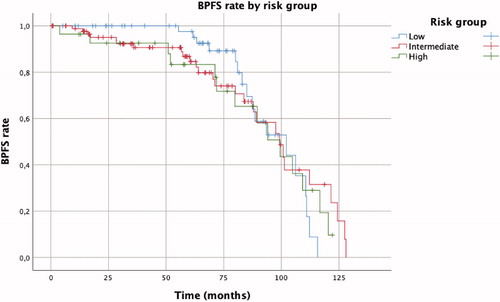
The mean follow-up was 60 months (SD 32.9). The Kaplan–Meier analysis () shows an overall BPFS rate was 67%. BPFS by risk group was 70.2%, 70.3% and 50.0% for the low, intermediate and high risk groups, respectively (p = 0.925). Overall time to BR was 93.67 months (SD 2.84, IC95%: 88.10–99.24): 95.91 (SD 3.44), 93.23 (SD 4.81) and 89.77 (SD 6.67) months for the low, intermediate and high risk groups, respectively. In both univariate and multivariate analysis the unic predictor for biochemical progression was de PSA nadir (HR 1.56, IC95%: 1.50–1.63), conferring an additional 4.56% risk for every 0.1ng/dL increase. 3-month hormonal therapy prior cryoablation was not a predictor of BR (p = 0.34). The mean post-treatment PSA nadir was 0.42 ng/dl (SD 1.56). Fifty-eight developed BR over time with a T mean PSA of 3.77 ng/ml (SD 3.09). Local recurrence was suspected in 40 cases (22.6%), and biopsy was performed in 34 out of them (66.7%) with confirmed tumor in 9 (69.3%). Recurrences were treated with radiotherapy in 6 cases, re-cryotherapy in 17 cases, hormone therapy in 14 cases, combined radiotherapy and hormone therapy in 8 cases and active surveillance in 13 cases. 15 patients died during follow-up for other causes than PC, no patient died of PC, thus the CSS was 100% and de OS 91.5%.
Functional outcomes
Full continence was maintained in 95% of patients after the procedure and only 1.7% reported UI (1 pad/day) after a year. Some grade of low urinary tract symptoms (LUTS) were reported in 13.5% of patients and were treated conservatively. All patients reported some grade of DE.
Discussion
The challenge of managing prostate cancer in the elderly is determining who will benefit from radical treatment. Historically, radical treatments such as RP and RT with or without ADT have been avoided as perceived as overtreatment, while ADT has been the gold-standard in older men with high-risk disease. However, the progressive ageing of the world male population, increasing comorbidity and crescent life expectancy is bringing out the need for tailored assessment in treatment of old PC patients. More important than the risk associated with advanced age, is the risk conferred by the increasing comorbidity [Citation13]. Both the International Society of Geriatric Oncology and the European Association of Urology (EAU) recommend that treatment decisions have to take into account not only chronological age, but the individual health status, risk of dying from PC and potential adverse effects [Citation1,Citation14].
Although cryotherapy has been a treatment option for over 30 years and it was approved by the Food and Drug Administration (FDA) in 2000, it is still considered by both the European Urological Association (EAU) an investigational therapy for localized PC with low level of evidence [Citation1]. The most extensive data and longest follow-up on whole gland cryotherapy as a treatment of localized PC come from the Cryo-On-Line Data (COLD) registry. This database is sponsored by the HealthTronics Corporation (Austin, TX, USA), but managed by an independent Board of Directors and housed in an independent research entity, Watermark Corp. (Indianapolis, IN, USA), in order to maintain the scientific integrity. Jones et al. [Citation15] reviewed a total of 1198 patients from the COLD registry, with clinically localized PC who underwent primary cryotherapy. With a mean follow-up of 24 months the actuarial 5-year BPFS using the Phoenix definition was 91.1%, 78.5% and 62.2%, for the low, moderate and high risk groups, respectively. From the same registry, Dhar et al. [Citation16] analyzed the results of whole-gland cryotherapy for PC treatment in older men (>75 years old). A total of 890 patients were included for analysis, the mean follow-up was 16 months and the actuarial 5-year BPFS rate using the Phoenix criteria was 74.9%, 61.4% and 58% for the low, moderate and high risk groups, respectively.
Prostate cryoablation is a minimal invasive technique conferring low morbidity profile. Patients are usually discharged the same day or the day after the procedure, representing a shorter hospitalization time than in a RP and may be preferred to the repeated hospital attendance needed when external beam RT is performed. Complications occurring after cryotherapy have been widely studied in small single hospital-based studies. The most frequent described early complications after cryotherapy are urinary tract infection (3–34%), perineal pain (0–12%) and urinary retention (3–23%) [Citation7]. Since the introduction of 3rd-generation cryosurgery equipment severe complications such as urethral sloughing and rectal fistula are anecdotal. The main long-term complications are LUTS, incontinence and ED [Citation7]. To de date, the impact of cryosurgery in LUTS remain unclear because the contradictory results reported in the literature that could be explained by information bias or further tracking during follow-up. In this study we report a LUTS rate of 13.5% based on patient self-reported symptoms within the first year of follow-up. We have previously analyzed urinary symptoms in 25 patients after primary cryotherapy. Those patients were evaluated using IPSS questionnaire, a three-day voiding diary (3 DVD) and uroflowmetry with ultrasound-measured post void residual volume (PRV), performed before, at 3, 6 and 12 months after the treatment [Citation17]. Rates of incontinence vary from 2 to 32% in the literature [Citation7]. Such a wide range reflects differences in terms of definition. Bahn et al. [Citation18] series reported a 16% of incontinence (any leak, even a drop). This percentage drop until 4.3% if incontinence was considered when any pads was used. Using the broad definition of any leakage/any drop, we obtained an incontinence rate of 5%, decreasing to 1.7% when the patient required the use of at least one pad per day. Potential resolution of incontinence over time can be explained because axonal regeneration. Some series reported a regained continence rates for those patients who were considered continent before surgery between 80 and 85% with an average recovery time around 6–9 months [Citation7]. ED is still a major complication after cryosurgery, since during the procedure, the surgeon freezes the apex of the gland and the periprostatic tissues where the neurovascular bundles are. Series reporting rates between 18 and 93% which also reflects differences in the definition used, information bias and follow-up [Citation7]. It has been reported around 50% of ED recovery within 2 years after cryosurgery [Citation19].
Cryotherapy energy can also be used for focal ablation of the prostate [Citation1]. Focal therapy is an emerging treatment option that involves the focal ablation of cancer tissue while preserving the surrounding healthy tissue, which might result in reduced morbidity when compared with whole-gland ablation therapies. The evolution of prostate imaging with high-quality multiparametric MRI have result in highly accurate disease localization and pathological confirmation due to the possibility of targeted biopsy [Citation20]. Despite the multifocal nature of PC in over 90% of patients, clinically significant disease has been previously defined as tumors with a volume of >0.5 cm3 and a Gleason score component >3 [Citation21,Citation22]. Focal therapy relies on the concept that residual prostate cancer in the untreated area does not compromise long-term disease control if clinically insignificant [Citation23]. Despite the EAU considers that focal therapy should only be performed within the context of a clinical trial setting or well-designed prospective cohort study, an expert consensus panel recommended the use of focal therapy in patients with a life expectancy of >5 years, a WHO performance status of 0 or 1 and intermediate-risk prostate cancer [Citation1,Citation20,Citation23]. Cryotherapy energy can also be used for focal ablation of the prostate. The available data indicate that focal cryotherapy seems to result in acceptable medium-term oncological outcomes with favorable morbidity profiles. The largest series on cryotherapy for prostate cancer to date was reported by Ward and colleagues based on data from the COLD Registry [Citation24]. This group reported outcomes of 1,160 patients after cryotherapy for localized prostate cancer and demonstrated a 3-year recurrence-free rate of 75.5% (according to ASTRO criteria), which is similar to that of whole‐gland cryoablation. Regarding functional outcomes, Ward et al. reported a fully urinary continence of 98.4%, which is similar to the whole‐gland cryoablation series, but the maintenance of spontaneous erections was 58.1% which is higher than those reported in whole‐gland cryoablation series. Werneburg et al. [Citation25] compared the functional results of focal versus total cryoablation of the prostate and concluded that focal cryotherapy and moderate-cold temperature were associated with favorable sexual function relative to total cryotherapy and very cold temperature, they did no find significant differences in urinary function or bowel habits either. We have recently started our focal therapy program for PC in highly selected patients, so further experience is needed in our center in order to evaluate its results.
The major limitation of the present study is retrospective nature. Another limitation is the disparate use of MRI for the diagnosis of local recurrence and follow-up. The lack of routinely performed standardized questionnaires that led our functional outcomes being patient-reported and physician-recorded, limiting their interpretation is another limitation to take into account. Finally, ED was not assessed before surgery and given the profile of our patients, the pretreatment ED might be high. Further, we did not track the evolution of erectile function over time.
Conclusion
In our series third generation cryotherapy systems have led to a more effective and safe procedure that offers competitive oncological results, low morbidity profile and a short treatment time in selected patients presenting localized prostate cancer and comorbidities. .
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mottet N, Bellmunt J, Briers E, et al. ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Edition presented at the EAU Annual Congress Barcelona. Arnhem (The Netherlands): EAU Guidelines Office; 2019.
- Ho JY, Hendi AS. Recent trends in life expectancy across high income countries: retrospective observational study. BMJ. 2018;362:k2562.
- Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107:576–584.
- Ries LAG, Melbert D, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975–2005. Bethesda (MD): National Cancer Institute; 2008.
- Everaerts W, Van Rij S, Reeves F, et al. Radical treatment of localised prostate cancer in the elderly. BJU Int. 2015;116:847–852.
- Sanda MG, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. AUA; 2017.
- Babaian RJ, Donnelly B, Bahn D, et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol. 2009;181:2388.
- D’Amico AV, Moul J, Carroll PR, et al. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Cin Oncol. 2003;21:2163–2172.
- Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974.
- Baust JG, Gage AA, Bjerklund Johansen TE, et al. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology. 2014;68:1–11.
- Escudero BA, Rodríguez P, Arias FF. Principios técnicos de criocirugía prostática (parte I). Archivos Españoles de Urología. 2003;56:1089–1109.
- Rodríguez SA, Arias Fúnez F, Bueno Bravo C, et al. Cryotherapy for primary treatment of prostate cancer: intermediate term results of a prospective study from a single institution. Prostate Cancer. 2014;2014:571576.
- Wirth MP, Froehner M. Co-morbidity in prostate cancer. Aging Male. 2000;3:132–136.
- Boyle HJ, Alibhai S, Decoster L, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116–136.
- Jones JS, Rewcastle JC, Donnelly BJ, et al. Whole gland primary prostate cryoablation: initial results from the cryo on-line data registry. J Urol. 2008; Aug180:554–558.
- Dhar N, Ward JF, Cher ML, et al. Primary full-gland prostate cryoablation in older men (> age of 75 years): results from 860 patients tracked with the COLD Registry. BJU Int. 2011;108:508–512.
- Mateu L, Peri L, Franco A, et al. Functional outcomes after prostatic cryosurgery. Act Urol Esp. 2018;42:338–343.
- Bahn DK, Lee F, Badalament R, et al. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urol. 2002; 60:3–11.
- Asterling S, Greene DR. Prospective evaluation of sexual function in patients receiving cryosurgery as a primary radical treatment for localized prostate cancer. BJU Int. 2009;103:788–792.
- Reis LO, Billis A, Zequi SC, et al. Supporting prostate cancer focal therapy: a multidisciplinary International Consensus of Experts ("ICE"). Aging Male. 2014;17:66–71.
- Villers A, McNeal JE, Freiha FS, et al. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992;70:2313–2318.
- Wise AM, Stamey TA, McNeal JE, et al. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–269.
- Perera M, Krishnananthan N, Lindner U, et al. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13:641–653.
- Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU Int. 2012;109:1648–1654.
- Werneburg GT, Kongnyuy M, Halpern DM, et al. Effects of focal vs total cryotherapy and minimum tumor temperature on patient-reported quality of life compared with active surveillance in patients with prostate cancer. Urology. 2018;113:110–118.