Abstract
Secondary spinal cord changes can follow spinal cord injuries (SCIs). This retrospective study was to uncover the chronic secondary changes that affect the spinal cord following severe injuries and to evaluate the influence of residual spinal deformity in the development of posttraumatic spinal cord changes. Fifty-eight patients (39 male, 19 female) with complete traumatic SCI and recent Magnetic resonance imaging (MRI) follow-up were reviewed retrospectively. A minimum of 2 years duration between trauma and MRI study was required (mean 2.9 years [2.1–4.7]). Two groups of patients were formed: with spinal deformity (and or spinal canal compromise) and without spinal deformity (and or spinal canal compromise). MRI of the injured spine demonstrated four major types of spinal cord changes; these are spinal cord atrophy, myelomalacia, syrinx, and focal cyst formation. The correlation of these changes to the presence of spinal deformity and or spinal canal compromise was also studied. Twenty-three patients (40%) of the studied population had more than 30° kyphosis and or 50% compromise of the spinal canal. Chronic spinal cord changes occurred in 25 patients (43%), 17 of these changes occurred in patients with spinal deformity and the remaining 8 occurred in patients without spinal deformity or canal compromise (p ≤ .05). The prevalence of spinal cord atrophy and focal cysts was significantly higher in patients with residual deformity and or spinal canal compromise (p ≤ .05). The authors recommend proper spinal cord decompression and fixation for patients with complete SCI to reduce the chance of secondary SCI.
Introduction
The improvement of medical services for patients with spinal cord injuries (SCI) in the last few decades played a major role in reducing the morbidity and mortality for this group of patients, currently the lifespan for SCI patients approaching those of normal population, this enabled researchers to study chronic spinal cord changes following SCI. Magnetic resonance imaging (MRI) can clearly show the changes that affect the spinal cord [Citation1]. The availability of MRI-compatible spinal fixation systems made of titanium had enabled us to study the changes that involve the spinal cord in patients who underwent instrumentation of the spine following traumatic spinal injury [Citation2].
Different reports described the secondary changes that affect the spinal cord following traumatic injury [Citation2–5]. Wang et al. studied these changes in 153 in patients who had sustained a SCI by MRI scanning; they reported four types of spinal cord pathologies in order of prevalence (extended atrophy, malacia, syrinx, and focal cyst) [Citation5]. Yamashita et al. similarly described these changes in 48 patients with chronic SCI [Citation2].
The clinical implications of these changes in patients with complete SCI are not clear yet. Wang et al. reported worsening pain and spasticity in patients who developed myelomalacia, cord atrophy, and traumatic syrinx but not in patients with focal cysts [Citation5]. Lee et al. similarly reported pain as the most frequent manifestation for PPTM, followed by motor deterioration and spasticity [Citation6]. As these changes can worsen the quality of life in these injured group of patients and its treatment are not well understood, the authors studied the role of spinal deformity and spinal canal compromise in the formation of these secondary spinal cord changes. The main aim of this report is to check whether proper decompression, reduction, and instrumentation following SCI can prevent the occurrence of these secondary changes.
Methods
Complete traumatic spinal cord injured patient recent MRI follow-up at King Abdullah University Hospital were reviewed retrospectively. A minimum of 2 years duration between trauma and MRI study was required (mean 2.9 years [2.1–4.7]). The patient's age, sex, mechanism of injury, time from injury, and injured segment were analyzed. All patients had undergone recent CT scan and or MRI to the injured segment of the spinal column as part of clinical follow-up.
Based on CT scan the authors identified two groups of patients based on the presence of residual kyphosis (>30°) and or spinal canal compromise (>50%). Using the MRI the authors focused on four types of secondary changes affecting the spinal cord following complete SCI: atrophy, myelomalacia, syrinx, and focal cyst formation. Combinations of these changes are frequently seen.
Spinal cord atrophy is defined as abnormal narrowing of the spinal cord in the sagittal plane (7 mm in the cervical and 6 mm in the thoracic cord) two segments or more beyond the limits of the vertebral injury (). Myelomalacia is seen on MRI as an area of low T1W and high T2W signal intensity that is between that of normal spinal cord and the surrounding cerebrospinal fluid (CSF) with ill-defined contours and irregular shapes (). Syrinx is an area with the same signal intensity as CSF which is usually tapered at one or both ends and which may appear loculated, with a well-defined contour and extends beyond the length of maximal bony damage (). Focal cyst is an area having the same signal intensity and contour definition as syrinx, with a round or oval shape and confined to the site of maximal bony protrusion into the spinal canal ().
Figure 1. T2W MRI, sagittal view of the dorsal spine, of a 22-year-old male involved in a motor vehicle accident 2 years ago. He presented with fracture dislocation of D12–L1 vertebrae. The patient was paraplegic, ASIA A score. The follow-up MRI shows the failure of instrumentation resulting in severe kyphosis and obliteration of the spinal canal which is associated with spinal cord atrophy.
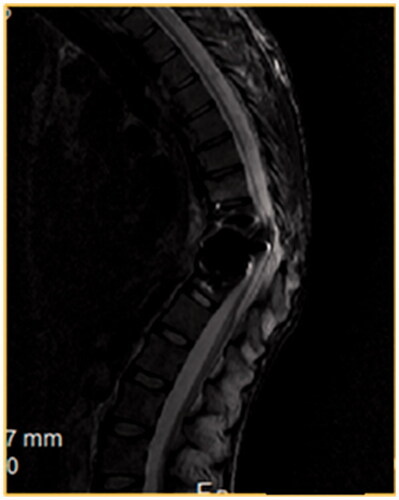
Figure 2. A T2W MRI, a sagittal view of the cervical spine, of a 35-year-old male patient victim of MVA that resulted in fracture C6–C7. The patient had ASIA A score. One year after the initial injury he presented with myelomalacia.
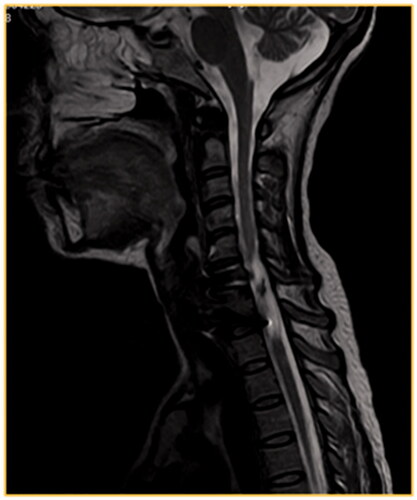
Figure 3. A T1W MRI image, sagittal view of the cervical spine, of a 28-year-old male involved in MVA that resulted in the fracture of D6, ASIA A, no surgical intervention. After 2 years, follow-up MRI showed syringomyelia that extended to the cervical spinal cord.
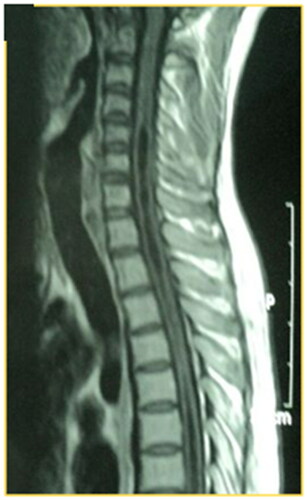
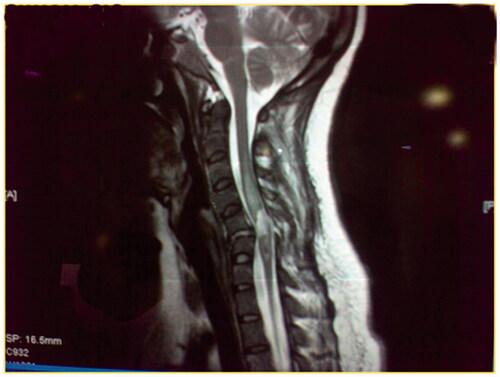
Figure 4. A T2W MRI image of a 25-year-old male involved in MVA that resulted C5–C6 fracture, classified as ASIA A, no surgical intervention. After 15 months he developed s focal cyst opposite to the fracture site.
The authors studied the prevalence of these spinal cord changes in the studied patients and correlate these changes to the presence of spinal deformity and or compromise of the spinal canal. This article is compliant with ethical standards. It was not funded from any source. No conflict of interest. All patients’ data are kept confidential and in compliance with the declaration of Helsinki.
Statistical analysis
Patient characteristics were summarized using descriptive statistics, which were expressed as the mean ± SD for continuous variables and percentages for categorical variables. Comparisons of age, gender, mechanism of injury, time from injury, and injured segment between patients with stable or unstable spine groups were performed using independent sample T-test. p Values ≤ .05 were considered significant.
Results
Fifity-eight patients (39 males, 19 females) with complete SCI were included. The mean age was 27.3 ± 5.2 years (range18–46 years). Sixteen of the injuries were in the cervical and 42 affected the dorsal spine. The time of assessment was 25–56 months from the time of injury. Road traffic accident was the mechanism of injury in 40 patients and falling down in the remaining 18 patients.
All except five patients underwent surgical stabilization. Spinal deformity with or without spinal canal compromise was found in 23 patients (40%), the remaining 35 patients (60%) had no spinal deformity or spinal canal compromise. Spinal deformity was found to be due to nonsurgical management in 4 patients, incomplete decompression and reduction in 11 and incomplete fixation in 5 patients. Spinal canal compromise was found in 12 patients. There is no significant difference between the two groups (with and without deformity) regarding sex, age, mechanism of injury, duration of the injury, and the involved segment of the spinal cord (thoracic versus cervical spine). summarizes the patients' demographic data and compares patients with and without spinal deformity.
Table 1. Patients demographic data and spinal deformity comparison. Significant data (p ⩽ .05) are marked in bold.
About 17 (74%) out of 23 patients with a spinal deformity or canal compromise showed spinal cord changes on the MRI. While only 8 (23%) of the 35 patients without spinal deformity or canal compromise showed spinal cord changes (p ≤ .05). Secondary spinal cord changes and its distribution among the two groups are summarized in . Spinal cord atrophy was found in seven patients with a spinal deformity or canal compromise and three patients without spinal deformity (p ≤ .05). Similarly, the occurrence focal cyst was significantly higher in spinal deformity patients group (p ≤ .05). The occurrence of myelomalacia and syringomyelia was more frequent in patients with spinal deformity and or spinal canal compromise.
Discussion
Traumatic SCI worldwide prevalence is approximately 750 per million with neurologic complications in 15–30% of patients, the most common etiology being motor vehicle accidents (40–45%), followed by falls (15–30%), sports injuries (15–25%), occupational injuries and assaults [Citation7,Citation8]. SCI is associated with high rates of morbidity and mortality especially in the elderly or the multiple-injured patients [Citation9].
There is a consensus that classes B–D on ASIA scale can achieve neurologic recovery if surgery is carried out early, preferably within 6 h of the initial trauma [Citation10,Citation11]. Many quadriplegics or paraplegic patients after SCI remain neurologically stable for long times, but a proportion will deteriorate over a variable time from injury and develop secondary injuries known collectively as posttraumatic progressive myelopathy (PTPM) [Citation1]. While the cause for these secondary myelopathies was believed to be metabolic such as hypoxia and oxidative damage, there is no data concerning the detection of its consequences on MRI. The authors believe that the posttraumatic progressive myelopathy can be explained by the changes in MRI described here.
In the past, PTPM could not be diagnosed with myelography or computed tomography myelography (CTM). Recently, advanced magnetic resonance (MR) imaging with higher contrast resolution, bony artifacts filtration, and multiplanar and multiphase capabilities has become the diagnostic imaging of choice. MRI can identify secondary spinal cord lesions in patients with long-standing spinal trauma, such as cord atrophy and myelomalacia [Citation2]. Sagittal views serve the most important diagnostic data, while the axial images serve an auxiliary role [Citation12].
When the initial treatment of SCI is inadequate, such as inadequate decompression, reduction or inadequate fixation, it can result into the deformity of the spine and spinal canal compromise leading to frequent micro-injuries and microhemorrhages that cause expansion of the cord cyst or cavity (post-traumatic syringomyelia or progressive post-traumatic cystic myelopathy) which results in progression of neurologic deficit [Citation4]. In our series, syrinx and or focal cyst represent 40% of secondary spinal cord changes, the majority had a spinal deformity.
The commonest MRI findings in patients with post-traumatic progressive myelopathy are cord atrophy, the most frequent clinical presentations of cord atrophy are progressive or ascending myelopathy following clinically stable injury [Citation6]. Spinal atrophy is usually seen after a long history (more than 2 years) of post-traumatic myelopathy [Citation4]. Extended atrophy is the most common abnormality seen in patients imaged years after SCI with a prevalence of 62%. In our series, SC atrophy represents 40% of secondary spinal cord changes, this lower incidence can be explained by the short duration of SC injury in our report compared to other reports.
Post-traumatic syringomyelia can occur anywhere between 3 months and 34 years following the primary SCI with an incidence of 3.4% [Citation13,Citation14]. Symptoms associated with syringomyelia formation include pain, motor deficit, sensory and temperature sensation changes, loss of reflexes, and increased spasticity [Citation13]. Syringomyelia formation is twice common in the complete SCI than incomplete injury [Citation4]. A spinal cord cyst is most commonly associated with cervical spine injuries with a prevalence of 9% in patients with chronic SCI [Citation5]. The prevalence of these chronic changes in the current report complies with previous reports.
Because the development of these chronic changes deteriorates the neurological status following a stable course of SCI victims, its prevention is essential to preserve the clinical course of those patients. However, it’s still not well understood how and why these chronic changes occur. This report demonstrates that proper surgical decompression, reduction, and fixation can significantly reduce the chance of secondary spinal cord changes following SCI.
Controversy still exists whether spinal fixation after complete SCI and class A ASIA patients has potential benefits regarding the neurologic and functional outcomes. Some authors documented no benefit of early fixation if paraplegia is complete [Citation15–18]. While others considered early surgery to be without neurologic benefit except reducing postoperative complications and hospital stay [Citation19]. In contrast, proponents of early stabilization documented the promotion of neurologic recovery [Citation20,Citation21]. La Rosa et al. in a meta-analysis review suggested that spinal decompression and fixation within 24 h resulted in improved neurologic outcomes in comparison to both delayed decompression and medical treatment, even for patients with complete spinal injury the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) represents the largest international, prospective multi-center study comparing early versus late surgical decompression for acute traumatic SCI [Citation22]. Unadjusted analysis showed a significant difference in the proportion of patients recovering at least two ASIA grades at 6 months’ follow-up in favor of the early intervention group [Citation23].
The authors emphasize the role of early surgery as it may change the neurological outcome of these young patients and can prevent the secondary spinal cord changes that may worsen the neurological function in the long term as described in this report.
Conclusions
The secondary sequelae to SCI are well-recognized, and there is a need for more significant controlled trials to write down guidelines for early surgical decompression and fixation and refine the controversy. The authors recommend early surgical intervention, even within 72 h, as a preventive measure against secondary SCI effects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gebarski SS, Maynard FW, Gabrielsen TO, et al. Posttraumatic progressive myelopathy. Clinical and radiologic correlation employing MR imaging, delayed CT metrizamide myelography, and intraoperative sonography. Radiology. 1985;157(2):379–385.
- Yamashita Y, Takahashi M, Matsuno Y, et al. Chronic injuries of the spinal cord: assessment with MR imaging. Radiology. 1990;175(3):849–854.
- Tator CH. Pathophysiology and pathology of spinal cord injury. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. 2nd ed. New York, NY: McGraw- Hill; 1996. 2847–2859.
- Curati WL, Kingsley DP, Kendall BE, et al. MRI in chronic spinal cord trauma. Neuroradiology. 1992;35(1):30–35.
- Wang D, Bodley R, Sett P, et al. A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia. 1996;34(2):65–81.
- Lee TT, Alameda GJ, Gromelski EB, et al. Outcome after surgical treatment of progressive posttraumatic cystic myelopathy. J Neurosurg. 2000;92(2 Suppl):149–154.
- Wyndaele M, Wyndaele J-J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44(9):523–529.
- Holmes JF, Miller PQ, Panacek EA, et al. Epidemiology of thoracolumbar spine injury in blunt trauma. Acad Emerg Med. 2001;8(9):866–872.
- McHenry TP, Mirza SK, Wang J, et al. Risk factors for respiratory failure following operative stabilization of thoracic and lumbar spine fractures. J Bone Joint Surg Am. 2006;88:997–1005.
- Fehlings MG, Perrin RG. The timing of intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31:S28–S35.
- Fehlings M, Rabin D, Sears W, et al. Current practice in the timing of surgical intervention in spinal cord injury. Spine. 2010;35:166–173.
- Mcardle CB, Crofford MJ, Mirfakhraee M, et al. Surface coil MRI of spinal trauma: preliminary experience. Am J Neuroradiol. 1986;7:885–893.
- Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. 1996;60(1):61–67.
- El Masry WS, Biyani A. Incidence, management, and outcome of post-traumatic syringomyelia. In memory of Mr Bernard Williams. J Neurol Neurosurg Psychiatry. 1996;60(2):141–146.
- Rahimi-Movaghar V, Vaccaro AR, Mohammadi M. Efficacy of surgical decompression in regard to motor recovery in the setting of conus medullaris injury. J Spinal Cord Med. 2006;29(1):32–38.
- Papadopoulos SM, Selden NR, Quint DJ, et al. Immediate spinal cord decompression for cervical spinal cord injury: feasibility and outcome. J Trauma. 2002;52(2):323–332.
- Vaccaro AR, Daugherty RJ, Sheehan TP, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:2609–2613.
- Waters RL, Adkins RH, Yakura JS, et al. Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord. 1996;34(4):188–192.
- McKinley W, Meade MA, Kirshblum S, et al. Outcomes of early surgical man-agement versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85(11):1818–1825.
- Cengiz SL, Kalkan E, Bayir A, et al. Timing of thoracolumbar spine stabilization in trauma patients; impact on neurological outcome and clinical course. Areal prospective (rct) randomized controlled study. Arch Orthop Trauma Surg. 2008;128(9):959–966.
- Dendrinos GK, Halikias JG, Krallis PN, et al. Factors influencing neurological recovery in burst thoracolumbar fractures. Acta Orthop Belg. 1995;61(3):226–234.
- La Rosa G, Conti A, Cardali S, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42(9):503–512.
- Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PloS One. 2012;7(2):e32037.
