Abstract
Several questionnaires have been developed to assist the diagnostic process in obstructive sleep apnea syndrome (OSAS). Berlin Sleep Questionnaire (BSQ) represents a validated screening tool for OSAS. Totally 450 patients admitted to the Sleep Center at Dicle University Medical Faculty were included prospectively. A risk analysis was performed for presence of OSAS using the BSQ. Arterial blood gas measurements were performed including bicarbonate (HCO3) level. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of BSQ for presence of OSAS and severe OSAS were determined. In patients with arterial HCO3 >24.94 mEq/L; sensitivity, specificity, PPV and NPV, of the BSQ were 93.04, 57.1, 98.3, and 23.5%, respectively. The addition of arterial HCO3 value increased the sensitivity of the BSQ in detecting OSAS patients. Although the cost of sleep studies is high for false positives from the BSQ plus arterial HCO3 level, this cost should be compared with the loss of work efficiency and severe healthcare costs of undiagnosed cases in the future. Therefore, finding possible OSAS cases in primary care health centers is important and adding serum HCO3 value to BSQ questionnaire may contribute to this topic.
Introduction and objective
Obstructive Sleep Apnea Syndrome (OSAS) is the most common type of sleep disordered breathing that characterized by episodes of partial or total upper airway obstruction during the sleep [Citation1,Citation2]. The estimated prevalence is approximately 15–30% in males and 10–15 in females [Citation2]. The prevalence of OSAS increases from young adulthood through the 6–7th decade and approximately 2–3 times more common in males than females; however, risk seems to be similar in postmenopausal woman [Citation3]. Although the definitive diagnosis and classification of patients with OSAS is based on polysomnography (PSG) technique, unfortunately access to this diagnostic procedure is limited. Polysomnography is a costly diagnostic method and low number of specialized sleep clinics harbor long patient appointment lists. Detailed questions that explore the etiologies of daytime sleepiness may help clinicians in differential diagnosis. Several questionnaires have been developed to assist predicting OSAS in the primary care setting [Citation4]. Among these, Berlin Sleep Questionnaire (BSQ) represents a validated screening tool for OSAS [Citation5]. This questionnaire categorizes patients as either high or low risk for OSAS based on self reports of patients. Berlin Sleep Questionnaire has shown a high sensitivity in screening for the disease but poor specificity in the diagnosis [Citation5]. Many previous studies validated its use in predicting OSAS with comparing against PSG and reported various sensitivity and specificity values [Citation4]. Apnea and hypopnea episodes associated with OSAS have the potential to lead to intermittent hypoxia as well as hypercapnia, with compensatory reduction of renal bicarbonate (HCO3) excretion and resultant HCO3 retention [Citation6]. The Apnea hypopnea index (AHI) and OSAS severity correlate with serum HCO3 levels in arterial blood gas measurements [Citation7]. The association between arterial blood gas HCO3 values and OSAS is little studied yet. There is a growing interest in screening the general population, primary care patients, and other clinical populations for OSAS risk due to the negative consequences of untreated OSAS on health. Undiagnosed cases may arise the risks of hypertension, vascular diseases, as well as metabolic and endocrine disorders. The risk of motor vehicle accidents that untreated OSAS cases can cause is a separate social problem. Therefore, we decided to investigate whether incorporation of serum HCO3 level to BSQ will improve the diagnostic sensitivity and specificity of this tool.
Materials and methods
Patient selection
Patients admitted to the Sleep Disorders Center of Dicle University Medical Faculty between October 31, 2018 and April 30, 2019 for polysomnography examination and accepting study participation were included. The study protocol was approved by the Ethics Committee for Non-Interventional Research, in Dicle University Medical Faculty. All patients gave their written consent for participation in the study. Age, sex, information on history of chronic disease, use of antihypertensive agents and other drugs, snoring, witnessed apnea, excessive daytime sleepiness, smoking habits, and alcohol consumption was recorded. A risk analysis was performed for presence of OSAS by using the BSQ. Also, the daytime excessive sleepiness scores were determined with Epworth Sleepiness Scale (ESS). Patients’ height, waist circumference, and neck circumference were recorded in centimeters and weight in kilograms. Daytime arterial blood samples for determination of blood gases, including, pH, bicarbonate (HCO3), partial arterial oxygen pressure (PaO2) and partial arterial carbon dioxide pressure (PCO2) were collected after the polysomnographic evaluation. Patients with chronic obstructive pulmonary disease and OHS were excluded based on information of a gold standard evaluation of respiratory function and a blood gas analysis. Patients with neuromuscular diseases, delirious, renal failure or have a history of drug use associated with metabolic acidosis or alkalosis were excluded. Of the 450 participants, 115 and 32 were excluded, due to the absence of arterial blood gas measurement and/or inadequate sleep efficiency, respectively.
Polysomnography procedure
Electroencephalography (EEG), electrooculography (EOG), submental and pretibial electromyography (EMG), pulse oximetry, electrocardiography (ECG), thoraco-abdominal respiratory effort, oro-nasal airflow, snoring sound, pulse, and body position recordings were performed using a 44-channel, digital-video equipped polysomnography device (Compumedics, 2007) using the international 10–20 electrode system. A sleep technician attended the whole recording procedure in all patients. Patients were classified into the following groups based on the results of PSG; simple snoring: AHI <5 per hour, mild OSAS: AHI ≥5, but <15 per hour, moderate OSAS: AHI ≥15, but <30 per hour, severe OSAS: AHI >30 per hour.
Statistical analyses
Statistical analyses were performed using SPSS (Statistical Package for Social Sciences) software, version 22 (SPSS Inc., Chicago, IL, USA). Demographic, clinical data and polysomnographic and anthropometric measurements were evaluated. Kolmogorov–Simirnov test was used to determine the distribution of the data. Patients were categorized as OSAS group and non-OSAS group, based on PSG. Student’s t-test or Mann–Whitney U test was used to compare continuous variables in case of normal and non-normal distribution of data, respectively. The categorical variables were compared with the Chi-square test. Determinants of OSAS and their independent effects were investigated with Binary logistic regression analysis. A p value <0.05 was considered statistically significant. The, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the BSQ was estimated with cross-table method. Serum HCO3 levels were compared between OSAS and non-OSAS groups with independent T test. ROC curve analysis done to identify the best cut-off value for serum HCO3 in predicting OSAS patients. Then, the sensitivity, specificity, PPV, and NPV of BSQ in predicting OSAS patients were re-measured among patients in whom serum HCO3 level was above the cut-off value. Thus, the contribution of the arterial HCO3 measurements in improving the diagnostic yield of BSQ alone was identified. Similarly, the sensitivity, specificity, PPV, and NPV of the BSQ in identifying severe OSAS patients was tested with the cross-tab method. Serum HCO3 levels were compared using the independent T test between patients with or without severe OSAS. ROC analysis was used to determine the best cut-off value of serum HCO3 for predicting severe OSAS (). Finally, the sensitivity and the specificity of BSQ in predicting severe OSAS among patients in whom HCO3 were above that cut-off value. Therefore, contribution of HCO3 in improving the ability of the BSQ in identification of severe OSAS patients was estimated.
Results
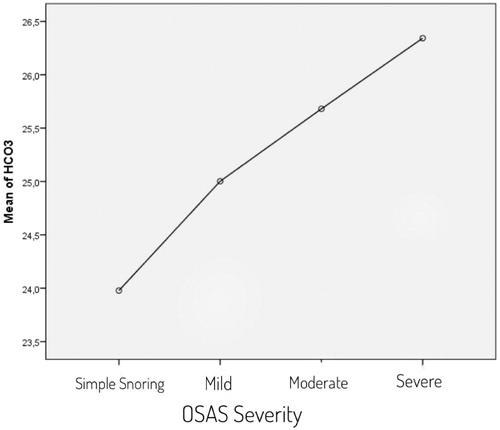
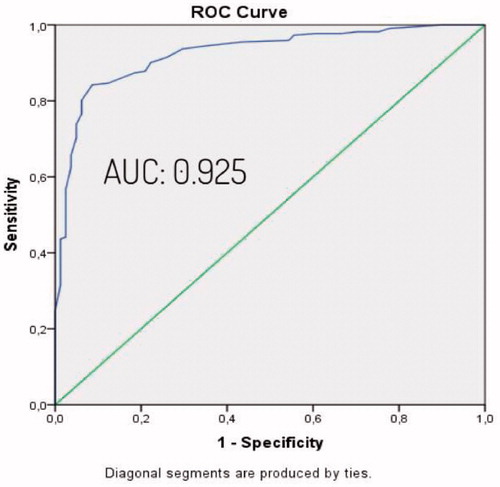
The study was conducted with the inclusion of a total of 303 eligible patients and 193 (63.7%) of patients were male and 110 (36.3%) were female. The mean age was 48.5 ± 12.6. Descriptive characteristics of patients are listed in . Demographic data were examined and weight, age, BMI and waist circumference were observed higher in the OSAS group. The difference was statistically significant. Arterial blood gas values were examined, and HCO3 value was higher and PaO2 value was lower in OSAS group (). To determine the independent factors that determine OSAS and the effect levels of these factors, all factors previously found significant in the Chi-square and Student t test were evaluated by binary logistic regression analysis. According to the BSQ, the high-risk group was predicted OSAS 11.85 times more compared to the low-risk group. It was observed that the probability of OSAS increased by 13.5 times for each unit arterial HCO3 level that increased. Other factors, which were found to be significant in the Chi-square and T test, did not have an independent and significant contribution in detecting OSAS (). Assessment of the polysomnography and arterial blood gas results by One-way Anova test showed that when OSAS severity increased, HCO3 levels increased in parallel ( and ). Pearson correlation test showed a correlation between AHİ and HCO3 level (r = 0.539, p = 0.000). Cross-tables were used to estimate the sensitivity, specificity, PPV and NPV of BSQ in identifying OSAS patients (). After significant differences in HCO3 levels were determined between patients with and without OSAS using the independent T test, the best cut-off value for serum HCO3 for predicting OSAS patients was found to be 24.94 mEq/L based on a ROC Curve analysis (AUC: 0.925) (). The sensitivity and the specificity of the BSQ in predicting OSAS patients were re-evaluated among patients with a HCO3 cut-off value above that level. Thus, the diagnostic contribution of the inclusion of HCO3 to the use of BSQ alone was determined (). Cross-tables were used to identify the sensitivity, specificity, PPV, and NPV of BSQ in identifying severe OSAS patients (). An independent T test was used to compare HCO3 levels in those patients with or without severe OSAS. The best cut-off value for serum HCO3 for predicting severe OSAS patients was found to be 25.45 mEq/L based on a ROC curve analysis (AUC: 0.857) (). The sensitivity and the specificity of the BSQ in predicting severe OSAS patients were re-evaluated among patients with a HCO3 cut-off value for severe OSAS. Thus, the diagnostic contribution of the inclusion of HCO3 to the use of BSQ alone was determined in terms of the identification of severe OSAS patients ().
Figure 3. The value of serum HCO3 levels in identifying severe OSAS patients (ROC curve), (AUC: 0.857).
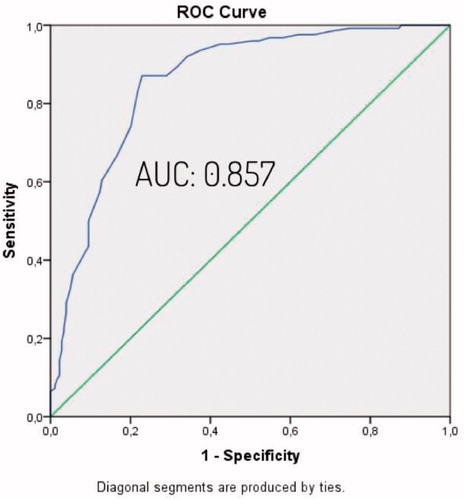
Table 1. Descriptive characteristics of patients.
Table 2. Comparison of demographic features and arterial blood gas values between patients with or without OSAS.
Table 3. Predictors of OSAS (binary logistic regression analysis).
Table 4. OSAS severity–HCO3 ANOVA.
Table 5. The predictive value of Berlin Sleep Questionnaire in identification of OSAS or severe OSAS patients and combination with HCO3 cut off values.
Discussion
In this cross-sectional study of a predominantly male patients without a chronic respiratory disorder, we established an association between wake arterial HCO3 concentration and AHI and therefore the severity of sleep apnea. According to the BSQ, the high-risk group was predicted OSAS 11.85 times more compared to the low-risk group. It was observed that the probability of OSAS increased by 13.5 times for each unit serum HCO3 level that increased. Cut of values for HCO3 were found for OSAS and severe OSAS patients in ROC analysis. The addition of arterial HCO3 increased the sensitivity of the BSQ in screening OSAS patients.
The increase in average lifespan globally as well as the growing population of middle-aged or elderly individuals, and increased prevalence of obesity have also led to an increase in OSAS prevalence [Citation8]. The effects of many comorbid medical conditions, concomitant drug use and age-related physiological changes in sleep architecture should be considered in evaluating the sleep of the elderly [Citation8]. Obstructive sleep apnea is an important treatable public health problem and associated with increased morbidity and mortality [Citation9,Citation10]. Treatment of patients with OSAS should not only aim to improve the patient’s sleep apnea, but also to improve the patient’s quality of life [Citation11]. Therefore, early diagnosis is crucial for prevention of the complications such as hypertension, serebrovasculer diseases and sexual dysfunction [Citation12,Citation13]. For all these reasons, methods detecting possible OSAS patients are very important. Although PSG represents the gold standard technique for the diagnosis of OSAS, it is a costly and time-consuming procedure. Increased awareness among general population regarding OSAS has resulted in long waiting lists in many sleep clinics, prompting investigators to search for OSAS screening tools [Citation14] and to develop several questionnaires aimed at identifying individuals at a high risk of OSAS. Among these, BSQ is a widely used clinical tool that has gone through robust clinical validation studies in epidemiological and clinical research [Citation5,Citation15]. A significant correlation between AHI and BSQ was found in the current study. The previous studies reported various sensitivity and specificity values for BSQ [Citation16,Citation17]. In a study by Kang et al., the sensitivity and specificity of the Korean version at a AHI cutoff of 5 were 69% and 83%, respectively [Citation14]. In another report involving 100 sleep clinics, Saleh et al. found a sensitivity and specificity of 97% and 90%, respectively, when an AHI cutoff of ≥ 5 was used [Citation18]. The corresponding figures reported by Sagaspe et al. at a AHI cutoff of 5 were 72% and 73%, respectively [Citation17]. The sensitivity of BSQ was high while its specificity was low in our study.
Arterial blood gas analysis provides valuable information regarding the metabolic and respiratory status of the patients. In a study involving patients with suspected OSAS, an independent association was found between serum HCO3 concentrations and the severity of sleep apnea [Citation19]. Also, other data showed that in a group of patients without chronic respiratory disorders, a definitive and dose-dependent association was present between OSAS and daytime arterial HCO3 concentrations [Citation7]. OSAS severity and HCO3 levels increased in parallel in our study. Recurrent upper airway obstruction in OSAS patients may lead to acute and intermittent hypercapnia during sleep. After each apneic episode, a hyperventilation response restores the CO2 concentrations [Citation20,Citation21]. Compensation fails when obstructive episodes become much longer and repetitive, and when excessive CO2 accumulation occurs. Furthermore, in some OSAS patients, the hyperventilation response between apneic episodes may be insufficient to eliminate the accumulated CO2. In theory, these two mechanisms may lead to CO2 accumulation during sleep and mild elevations in serum HCO3 levels, without causing significant daytime hypoventilation. Intermittent oscillations of oxygen and carbon dioxide (CO2) during the sleeping period is not only determined by altered ventilation during the apneic cycle, but also by the extent of tissue oxidative metabolism and tissue deposition of CO2 in the body [Citation22] Based on these, we hypothesized that increasing severity of OSAS may be associated with increased arterial HCO3 levels. In a previous study, in hypercapneic patients with severe OSAS, serum HCO3 levels were reported to be 32 mmol/L [Citation23]. Similarly, our patient population with OSAS also exhibited a significant increase in arterial HCO3 values. There is a growing interest in screening the general population, primary care patients, and other clinical populations for OSAS risk due to the negative consequences of untreated OSAS on health and the health costs of these results [Citation24,Citation25]. A previous study notified an association between multimorbidity and OSAS in a population of middle age to elderly men with undiagnosed OSAS [Citation26]. For this reason, we believe that there is further need for studies examining the practical diagnostic tests for OSAS. On behalf of a disease such as OSAS with severe consequences [Citation27,Citation28], high sensitivity to detect patients with sleep apnea bears more clinical significance than high specificity.
Although the cost of sleep studies is high for false positives from the BSQ plus arterial HCO3 level, this cost should be compared with severe healthcare costs of undiagnosed cases that may arise the risks of hypertension, vascular diseases, as well as metabolic and endocrine disorders, in the future. Undiagnosed each case carries risks for loss of productivity, poor quality of life and work-related accidents [Citation26,Citation29,Citation30]. Otherwise obstructive sleep apnea has been associated with a high risk for motor vehicle accidents. The cost of traffic accidents due to sleep apnea has been estimated to be so high. In our country and in many countries, driving applicants must be evaluated in terms of OSAS before they can be given a driver's license. Obviously, screening, diagnosing and then treating sleep apnea as soon as possible is very important for safe driving. Treating all drivers with sleep apnea would be cost saving compared to possible traffic accidents. Several population-based reports underscoring the need for a timely diagnosis and treatment. For all these reasons, finding possible OSAS cases in primary care health centers is important and adding serum HCO3 value to BSQ may contribute to this topic.
In conclusion, the use of BSQ in conjunction with serum HCO3 levels provided the highest sensitivity for OSAS presence. Determining the level of HCO3 in addition to BSQ in OSAS screening, may contribute to early detection of OSAS by physicians, and this may enable patients to be referred to a sleep center in the early period and may prevent possible complications. Such approach may allow for future reductions in costs of health and treatment.
There were some limitations of this study. This study was performed in a single center, and included a relatively small study population. Since the cases were patients admitted to the sleep center, we found a higher prevalence of OSAS (73.3%) than predictions from community-based questionnaires. In this study, the average BMI of the population was high. It is likely that the higher mean BMI of the sleep clinic population may have affected the sensitivity and the specificity of the BSQ as factors that greatly influenced the scoring of the Berlin questionnaire. Although our data has been validated internally, the results are from a single center and more studies are needed to apply the results of this study to other populations. There is still a need for further prospective, large-scale, controlled studies to evaluate the effects of using the HCO3 with BSQ.
Author contributions
Literature research: Mazlum Dursun and Hadice Selimoğlu Şen. Data collections: Mazlum Dursun, Hadice Selimoğlu Şen, and Süreyya Yılmaz. Study design: Mazlum Dursun, Hadice Selimoğlu Şen, Süreyya Yılmaz, and Gökhan Kırbaş. Analysis of data: Mahşuk Taylan and Hadice Selimoğlu Şen. Manuscript of preparation: all authors. Review of manuscript: all authors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yıldız Gülhan P, Güleç Balbay E, Elverişli MF, et al. Do the levels of particulate matters less than 10 μm and seasons affect sleep? Aging Male. 2020;23(1):36–41.
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- Tufik S, Santos- Silva R, Taddei JA, et al. Obstructive sleep apnea syndrome in the Sao Paulo epidemiologic sleep study. Sleep Med. 2010;11(5):441–446.
- Luo J, Huang R, Zhong X, et al. STOP-bang questionnaire is superior to Epworth sleepiness scales, Berlin questionnaire, and STOP questionnaire in screening obstructive sleep apnea hypopnea syndrome patients. Chin Med J (Engl). 2014;127(17):3065–3070.
- Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491.
- Norman RG, Goldring RM, Clain JM, et al. Transition from acute to chronic hypercapnia in patients with periodic breathing: predictions from a computer model. J Appl Physiol. 2006;100(5):1733–1741.
- Eskandari D, Zou Grote L, Schneider H, et al. Independent associations between arterial bicarbonate, apnea severity and hypertension in obstructive sleep apnea. Respir Res. 2017;18(1):130.
- Celikhisar H, Dasdemir Ilkhan G. Comparison of clinical and polysomnographic characteristics in young and old patients with obstructive sleep apnea syndrome. Aging Male. 2020;27:1–8.
- Gooneratne NS, Richards KC, Joffe M, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34(4):435–442.
- Shigehara K, Konaka H, Sugimoto K, et al. Sleep disturbance as a clinical sign for severe hypogonadism: efficacy of testosterone replacement therapy on sleep disturbance among hypogonadal men without obstructive sleep apnea. Aging Male. 2018;21(2):99–105.
- Balbay EG, Yildiz P, Elverisli MF, et al. The eating attitudes in patients with obstructive sleep apnea syndrome. Aging Male. 2020;12:1–6.
- Taken K, Ekin S, Arısoy A, et al. Erectile dysfunction is a marker for obstructive sleep apnea. Aging Male. 2016;19(2):102–105.
- Li X, Dong Z, Wan Y, et al. Sildenafil versus continuous positive airway pressure for erectile dysfunction in men with obstructive sleep apnea: a meta-analysis. Aging Male. 2010;13(2):82–86.
- Kang K, Park KS, Kim JE, et al. Usefulness of the Berlin Questionnaire to identify patients at high risk for obstructive sleep apnea: a population-based door-to-door study. Sleep Breath. 2013;17(2):803–810.
- Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin Questionnaire, STOP-BANG, STOP, and Epworth Sleepiness Scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57–70.
- Sharma SK, Vasudev C, Sinha S, et al. Validation of the modified Berlin questionnaire to identify patients at risk for the obstructive sleep apnoea syndrome. Indian J Med Res. 2006;124(3):281–290.
- Sagaspe P, Leger D, Taillard J, et al. Might the Berlin Sleep Questionnaire applied to bed partners be used to screen sleep apneic patients? Sleep Med. 2010;11(5):479–483.
- Saleh AM, Ahmad MA, Awadalla NJ. Development of Arabic version of Berlin questionnaire to identify obstructive sleep apnea at risk patients. Ann Thorac Med. 2011;6(4):212–216.
- Bingol Z, Pihtili A, Cagatay P, et al. Clinical predictors of obesity hypoventilation syndrome in obese subjects with obstructive sleep apnea. Respir Care. 2015;60(5):666–672.
- Berger KI, Goldring RM, Rapoport DM. Obesity hypoventilation syndrome. Semin Respir Crit Care Med. 2009;30(3):253–261.
- Berger KI, Ayappa I, Sorkin IB, et al. Postevent ventilation as a function of CO(2) load during respiratory events in obstructive sleep apnea. J Appl Physiol (1985). 2002;93(3):917–924.
- Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, et al. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11(2):117–124.
- Bahar Y, Annakkaya AN, Sen C, et al. Assessment of the frequency of deep venous thromboembolism in obstructive sleep apnea syndrome. Aging Male. 2019;22:1–6.
- Amra B, Nouranian E, Golshan M, et al. Validation of the Persian version of berlin sleep questionnaire for diagnosing obstructive sleep apnea. Int J Prev Med. 2013;4(3):334–339.
- Ruel G, Martin SA, Levesque JF, et al. Association between multimorbidity and undiagnosed obstructive sleep apnea severity and their impact on quality of life in mano ver 40 years old. Glob Health Epidemiol Genom. 2018;3:e10.
- Senaratna CV, Perret JL, Matheson MC, et al. Validity of the Berlin questionnaire in detecting obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:116–124.
- Durmaz DY, Güneş A. Is there a relationship between hematological parameters and duration of respiratory events in severe OSA. Aging Male. 2020;23(2):125–131.
- Bjornsdottir E, Keenan BT, Eysteinsdottir B, et al. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J Sleep Res. 2015;24(3):328–338.
- Egea Santaolalla CJ, del Campo Matias F. Work-related accidents, absenteeism and productivity in patients with sleep apnea. A future consideration in occupational health assessments? Arch Bronconeumol. 2015;51(5):209–210.