?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives
Vitiligo is an autoimmune disease, and its pathogenesis involves changes in cytokine levels in the affected patients. Tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-17 from pro-inflammatory cytokines, IL-37 in a recently detected anti-inflammatory activity. The aim of our study was to determine serum TNF-α, IL-6, IL-17, IL-37 levels in patients with vitiligo to understand their possible roles in the disease etiology and to compare the results with the healthy controls.
Methods
The study included 48 generalized vitiligo patients who were diagnosed with vitiligo, had an increase in the lesions within the last 3 months, and did not receive any systemic or topical treatment during this period; furthermore, 18 healthy controls were included.
Results
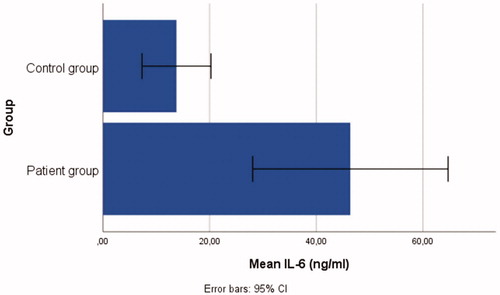
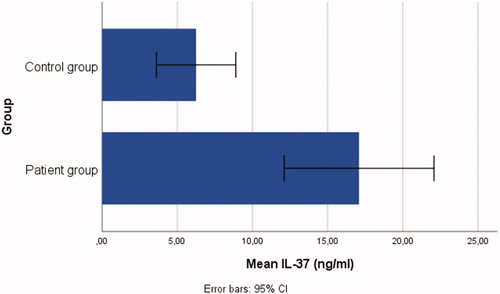
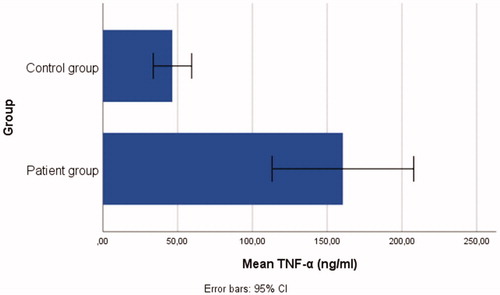
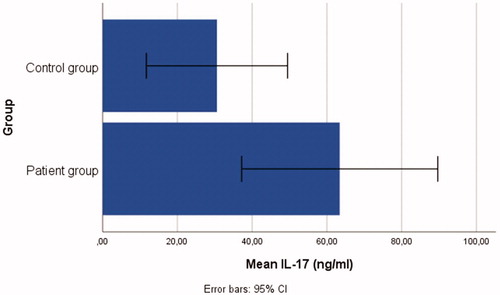
Patient group: n = 48, mean age = 30.48 ± 9.86 years; control group: n = 18, mean age = 28.27 ± 9.66 years. Individuals in the patient group had significantly higher serum levels of IL-37(t = 3.90, p < .001), IL-6 (t = 3.39, p < .05), IL-17 (t = 2.08, p < .05), and TNF-α (t = 4.69 p < .001) than in the control group.
Conclusion
The high levels of (pro-anti) inflammatory cytokines in vitiligo patients draw attention to the importance of cytokines in the pathogenesis of the disease.
Introduction
Vitiligo is a depigmentation disease of an unknown origin and is characterized by sharply demarcated white maculae developing as a result of melanocyte loss in the skin [Citation1]. Four theories of origin have been proposed to explain the pathogenesis of this disease: otocytotoxicity, autoimmune, oxidant–antioxidant (self-destruction), and neural theory leading to dermatomal melanocyte destruction [Citation2,Citation3]. Immune responses to Th1 or Th17 are induced in autoimmune diseases, and these responses may be responsible for the development and progression of the disease [Citation4]. It has been shown that CD4+ T cells play a crucial role in the pathogenesis of vitiligo. Naive CD4+ helper T cells are divided into four subtypes: Th1, Th2, Th17, and regulatory T cells (Tregs) [Citation5]. The major cytokines produced by Th1, Th2, and Th17 cells are important in the pathogenesis of the disease. Similar to pro-inflammatory cytokines, anti-inflammatory cytokines are also important in the pathogenesis of disease [Citation6]. IL-37 is an anti-inflammatory cytokine that was identified in recent years and is important in the pathogenesis of autoimmune diseases [Citation7]. However, very few studies have investigated the levels of pro-inflammatory–anti-inflammatory cytokines produced by Th1, Th2, and Th17 cells. Therefore, we conducted this study to evaluate the serum levels of these cytokines [TNF-alpha (α), IL-6, IL-17, and IL-37] in patients with vitiligo. We believe that the findings of our study will provide a better understanding of the pathogenesis of this disease.
Materials and methods
This prospective, case–control study was conducted at Dermatological and Venereal Diseases Outpatient Clinic of a tertiary hospital. The study included 48 generalized patients with vitiligo who were clinically diagnosed and confirmed with Wood's light examination, who had an increase in their lesions within the last 3 months, and who did not receive any systemic or topical treatment during this period—and 18 healthy controls (control group). The individuals in the control group was selected among those who agreed to participate in the study as healthy volunteers after considering the similarities with the patient group in terms of age and sex. In patients diagnosed with vitiligo, those with concomitant diabetes mellitus, pernicious anemia, and thyroid dysfunction were excluded from the study. The patient data for age, sex, and disease duration were recorded.
Venous blood samples were collected from the patient and control groups under sterile conditions. After centrifugation at 3000 rpm for 10 min, the serum was separated and the samples were stored at −80 °C until they were examined by ELISA.
The study was approved by the Ethics Committee (Duzce University Ethics Committee), and informed consent was obtained from each patient included in the study.
Elisa
Serum levels of were detected TNF-α, IL-6, IL-17, and IL-37 by enzyme-linked immunosorbent assay (ELISA). The double-antibody sandwich ELISA was used to assay the target protein level in the samples. The target protein was placed in an enzyme well, which was precoated with target protein monoclonal antibody, for incubation. Biotin-labeled antibodies were then added and combined with Streptavidin-HRP to form an immune complex. Following this, incubation and then washing was performed to remove the uncombined enzyme. Chromogen Solution A and B were then added; the color of the liquid changed to blue and finally became yellow due to the effect of acid. The chroma of the color and the concentration of the target protein in the samples were positively correlated [Citation8].
Statistical analysis
Prior to data analysis, descriptive statistics and analysis assumptions were examined. Standardized z-scores were used to detect outliers, and the results suggested that all standardized z-scores were within the acceptable range. Normality assumption was tested using skewness and kurtosis scores as well as their cut-off values. Descriptive statistics showed that all variables were relatively normally distributed. Independent samples t-test was performed to compare serum IL-37, IL-6, IL-17, and TNF-α levels in the control and patient groups. Significance was set at p <.05. Additionally, the effect size was interpreted using Cohen’s d, and its decision rules are as follows: <0.5 = small, 0.5–0.8 = medium, and ≥ 0.8 = large. All data analyses were performed using IBM SPSS version 25.
Results
The study sample was comprised of 66 individuals [patient group: n = 48; 25 (52.1%) female and 23 male (47.9%); age range = 16–52 years; mean age = 30.48 ± 9.86 years; control group: n = 18; 7 (38.9%) female and 11(61.1%) male; age range = 14–46 years; mean age = 28.27 ± 9.66 years]. Demographic and clinical characteristics of the patient and control groups are presented in .
Table 1. Demographic and clinical characteristics of the study sample.
Independent samples t-test was performed to investigate the differential effect of the patient and control groups on the IL-37, IL-6, IL-17, and TNF-α levels. Descriptive statistics and t-test results are presented in .
Table 2. Descriptive statistics and between-group t-test outcomes.
The t-test outcomes demonstrated that there were significant differences between the patient and control group: IL-37(t = 3.90, p < .001), IL-6 (t = 3.39, p < .05), IL-17 (t = 2.08, p < .05), and TNF-α (t = 4.69, p < .001), ranging from small-to-large effect sizes (d range = 0.47–0.98). Individuals in the patient group had significantly higher serum levels of IL-37 (= 17.09 ± 17.17), IL-6 (
= 46.40 ± 63.09), IL-17 (
= 63.43 ± 90.33), and TNF-α (
= 160.56 ± 163.10) than those in the control group, see . Bar graphs demonstrating the serum IL-6, IL-37, TNF-α, and IL-17 levels of the patient and control groups are shown in .
Discussion
In this study; serum levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-17) and anti-inflammatory cytokine (IL-37) were statistically significantly higher than the control group. 52.1% of vitiligo patients were female and mean disease duration was 115.54 ± 95.46 months. Pathogenic role of cytotoxic, autoreactive CD8+ T cells in vitiligo, it appears that CD4+ Tregs play an important role in preventing and controlling diseases [Citation4]. Cytotoxic CD8+ T cells have an important role in melanocyte destruction in patients with vitiligo, and therefore, they function as the effector arm of autoimmunity in vitiligo. The infiltration level of CD8+ T cells was found to be associated with disease severity [Citation9]. Specific CD8+ T cells have been shown to respond against melanocyte-specific protein (melan-A/MART-1), tyrosinase, and gp100 from antigens showing melanocyte-specific differentiation in peripheral circulation. CD8+ T-cell infiltration supporting the immunological process has been identified in biopsies obtained from the active margin of vitiligo lesions [Citation10]. There is also evidence suggesting that cytokines and CD4+ helper T cell-mediated immunity may play an important role in the pathogenesis of vitiligo [Citation2]. As stated above, naive CD4+ helper T cells are divided into four subtypes: Th1, Th2, Th17, and Tregs. Th1 cells primarily produce interferon (IFN)-gamma (γ) and TNF-beta (β); Th2 cells synthesize IL-4, IL-5, and IL-13; Th17 cells produce IL-17 and IL-6; and Tregs synthesize IL-10 and transforming growth factor (TGF)-β [Citation5]. A significant increase in the expression of inflammatory cytokines in the skin with lesion compared with the skin without lesion has been reported in patients with vitiligo [Citation6]. Studies evaluating IL levels in patients with vitiligo have been reported in the literature. The primary function of TNF-α in vitiligo is the regulation of the immune responses; therefore, it has a central effector function in the immunopathological mechanisms involved in vitiligo [Citation11]. TNF-α causes keratinocyte apoptosis, which induces an autoimmune response and leads to melanocyte loss [Citation2]. Studies have reported an increase in TNF-α levels in patients with vitiligo compared with the control group, thereby supporting the above finding [Citation2,Citation12]. However, some studies have also reported no significant difference in cytokine levels between the patient and control groups [Citation13]. Yu et al. have found that serum levels of TNF-α decrease in patients with vitiligo [Citation14]. However, the treatment efficacy is variable when anti-TNF agents are used for vitiligo, but the development of vitiligo has also been reported in patients using anti-TNF agents for other diseases [Citation15]. IL-6 and IL-17 are pro-inflammatory cytokines that play an important role in the pathogenesis of autoimmune diseases [Citation16,Citation17]. Zhou et al., Elela et al., and Zhen et al. have found that IL-17 levels were increased in patients with vitiligo and that they correlated with the involvement area of the disease and were important in the pathogenesis of vitiligo [Citation18–20]. Sushama et al. and Yang et al. have reported an increase in serum levels of IL-6 in patients with vitiligo [Citation2,Citation21]. Moreover, another study has reported an increase in the expression of both IL-6 and TNF-α in keratinocytes [Citation22].
Serum levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-17) in patients with generalized vitiligo with new lesions emerging in the previous three months were significantly higher than in a control group. These pro-inflammatory cytokines may have other effects apart from disease activity. Although this is controversial, testosterone is thought to reduce pro-inflammatory cytokines and increase anti-inflammatory cytokines [Citation23]. In the study, which included 669 vitiligo patients, 62.2% of the patients were female [Citation24]. In this study, vitiligo was more prevalent among women, at 52.1%. We think that vitiligo being also less common in males in this study may be due to the protective effect of testosterone. Diabetes regulation has been achieved with testosterone therapy in patients with hypogonadism + Type II Diabetes Mellitus [Citation25]. If testosterone levels are low in male patients with vitiligo and hypogonadism, the vitiligo may perhaps improve with testosterone therapy. This can be elucidated through further studies of the therapeutic efficacy of testosterone. Studies in the literature have also detected an increased risk of metabolic syndrome in vitiligo patients [Citation26–28]. Solak et al. detected high serum CRP levels in patients with generalized vitiligo [Citation29]. Metabolic syndrome and serum CRP levels are considered as independent risk factors for carotid atherosclerosis [Citation30]. Some studies have also shown that testosterone reduces the atherosclerotic process. Mohammed et al. reported that testosterone may be capable of preventing pre-atherosclerosis since it reduces pro-inflammatory cytokines [Citation23,Citation31]. Pro-inflammatory can lead to disease activation, and also to metabolic syndrome. We think that these patients should also be evaluated from that perspective.
The patients included in the present study were generalized patients with vitiligo who had an increase in their lesions in the last 3 months and who did not receive any treatment agent. The serum levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-17 were higher in these patients than in the healthy control group; this finding is consistent with those reported previously in the literature. It is highly possible that there are anti-inflammatory cytokines that suppress this pro-inflammatory process and reduce the progression of the disease. Therefore, our study also investigated IL-37, whose anti-inflammatory effect was recently identified to be important in the pathogenesis of various diseases. In recent years, it has been identified that IL-37 from the IL-1 family plays an important regulatory role in the development of various inflammatory diseases, autoimmune diseases, and tumors. IL-37 has a structure similar to that observed in the IL-1 family [Citation7]. The IL-1 cytokine family, including seven pro-inflammatory agonists (IL-1α, IL-1, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ) and four defined or putative antagonists [IL-1R antagonist (IL-1Ra), IL-36Ra, IL-37, andIL-38], exhibits an anti-inflammatory activity. IL-37 can suppress the innate immune response by reducing the production of pro-inflammatory cytokines, which are possibly induced by Toll-like receptor agonists [Citation32]. Yuan et al. have found that IL-37 levels increased in patients with rheumatoid arthritis and were correlated with disease activity [Citation33]. IL-37 levels were shown to be significantly higher in the plasma of patients with SLE than in healthy controls [Citation34,Citation35]. Teng et al. have found that increased IL-37 expression in psoriatic plaques significantly reduced the inflammatory process in mice and suggested that IL-37 is an effective immune regulator that functions by inhibiting the expression of pro-inflammatory cytokines in the development of psoriasis [Citation36]. In our study, the serum levels of IL-37 were also found to be high in patients with vitiligo, which is consistent with the findings reported for other autoimmune pathogenesis in the literature.
In conclusion: Increases in pro-inflammatory cytokines and anti-inflammatory cytokines have been determined in the pathogenesis of vitiligo. Cytokines are important in the onset and progression of the disease. They may therefore act as a biomarker for identifying patients with progressive disease requiring aggressive treatment. Our research will not only lead to a better understanding of the pathogenesis of vitiligo, but also opens further avenues for the development of specific immunological intervention for the treatment of this challenging disease
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–1212.
- Sushama S, Dixit N, Gautam RK, et al. Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of disease. J Cosmet Dermatol. 2019;18(1):337–341.
- Nicolaidou E, Mastraftsi S, Tzanetakou V, et al. Childhood vitiligo. Am J Clin Dermatol. 2019;20(4):515–526.
- Weaver CT1, Harrington LE, Mangan PR, et al. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–688.
- Basak PY, Adiloglu AK, Ceyhan AM, et al. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol. 2009;60(2):256–260.
- Gomes IA, de Carvalho FO, de Menezes AF, et al. The role of interleukins in vitiligo: a systematic review. J Eur Acad Dermatol Venereol. 2018;32(12):2097–2111.
- Jia H, Liu J, Han B. Reviews of interleukin-37: functions, receptors, and roles in diseases. Biomed Res Int. 2018;2018:3058640
- Vashist SK, Luong JHT. Handbook of immunoassay technologies. 1st ed. London: Elsevier;2018.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1):1–13.
- Steitz J, Wenzel J, Gaffal E, et al. Initiation and regulation of CD8 + T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004;83(11–12):797–803.
- Camara-Lemarroy CR, Salas-Alanis JC. The role of tumor necrosis factor-α in the pathogenesis of vitiligo. Am J Clin Dermatol. 2013;14(5):343–350.
- Mitra S, De Sarkar S, Pradhan A, et al. Levels of oxidative damage and proinflammatory cytokines are enhanced in patients with active vitiligo. Free Radic Res. 2017;51(11–12):986–994.
- Singh S, Singh U, Pandey SS. Serum concentration of IL-6, IL-2, TNF-α, and IFNγ in Vitiligo patients. Indian J Dermatol. 2012;57(1):12–14.
- Yu HS, Chang KL, Yu CL, et al. Alterations in IL-6, IL-8, GM-CSF, TNF-alpha, and IFN-gamma release by peripheral mononuclear cells in patients with active vitiligo. J Invest Dermatol. 1997;108(4):527–529.
- Webb KC1, Tung R, Winterfield LS1, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol. 2015;173(3):641–650.
- Singh RK, Lee KM, Vujkovic-Cvijin I, et al. The role of IL-17 in vitiligo: a review. Autoimmun Rev. 2016;15(4):397–404.
- Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141(2):125–139.
- Zhou L, Shi YL, Li K, et al. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment Cell Melanoma Res. 2015;28(3):324–329.
- Elela MA, Hegazy RA, Fawzy MM, et al. Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: a case- controlled study on eighty-four patients. Eur J Dermatol. 2013;23(3):350–355.
- Zhen Y, Yao L, Zhong S, et al. Enhanced Th1 and Th17 responses in peripheral blood in active non-segmental vitiligo. Arch Dermatol Res. 2016;308(10):703–710.
- Yang X, Yan L, Ha D, et al. Changes in sICAM-1 and GM-CSF levels in skin tissue fluid and expression of IL-6, IL-17 and TNF-α in blood of patients with vitiligo. Exp Ther Med. 2018;17:408–412.
- Li P, Ma H, Han D, et al. Interleukin-33 affects cytokine production by keratinocytes in vitiligo. Clin Exp Dermatol. 2015;40(2):163–170.
- Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–140.
- de Barros JC, Machado Filho CD, Abreu LC, et al. A study of clinical profiles of vitiligo in different ages: an analysis of 669 outpatients. Int J Dermatol. 2014;53(7):842–848.
- Haider KS, Haider A, Saad F, et al. Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes Obes Metab. 2020:1–14.
- Tanacan E, Atakan N. Higher incidence of metabolic syndrome components in vitiligo patients: a prospective cross-sectional study. An Bras Dermatol. 2020;95(2):165–172.
- Pietrzak A, Bartosińska J, Hercogová J, et al. Metabolic syndrome in vitiligo. Dermatol Ther. 2012;25 Suppl 1 (Suppl 1):S41–S3.
- Ataş H, Gönül M. Increased risk of metabolic syndrome in patients with vitiligo. Balkan Med J. 2017;34(3):219–225.
- Solak B, Dikicier BS, Cosansu NC, et al. Neutrophil to lymphocyte ratio in patients with vitiligo. Postepy Dermatol Alergol. 2017;34(5):468–470.
- Kawada T, Otsuka T, Endo T, et al. Aging, components of metabolic syndrome and serum C-reactive protein showed significant relationship with carotid atherosclerosis. Aging Male. 2012;15(1):42–47.
- Rezanezhad B, Borgquist R, Willenheimer R, et al. Association between serum levels of testosterone and biomarkers of subclinical atherosclerosis. Aging Male. 2018;21(3):182–186.
- Palomo J, Dietrich D, Martin P, et al. The interleukin (IL)-1 cytokine family-Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76(1):25–37.
- Yuan ZC, Wang JM, Huang AF, et al. Elevated expression of interleukin-37 in patients with rheumatoid arthritis. Int J Rheum Dis. 2019;22(6):1123–1129.
- Wu G-C, Li H-M, Wang J-B, et al. Elevated plasma interleukin-37 levels in systemic lupus erythematosus patients. Lupus. 2016;25(12):1377–1380.
- Song L, Qiu F, Fan Y, et al. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J Clin Immunol. 2013;33(1):111–117.
- Teng X, Hu Z, Wei X, et al. IL-37 ameliorates the inflammatory process in psoriasis by suppressing proinflammatory cytokine production. J Immunol. 2014;192(4):1815–1823.