Abstract
Low baseline testosterone level has been associated with the development of risk factors for cardiovascular disease such as insulin resistance and obesity. In addition to the absolute testosterone level, remarkable changes in testosterone level may have an acute effect on cardiovascular disease development and progression, which has been rarely investigated. In this study, we used a clinical dataset of 376 hypogonadal men whose testosterone levels were measured every six months for up to 11 years from a registry study in Germany, and conducted survival analyses to investigate the effect of testosterone changes since the last visit (time-varying) on the risk of cardiovascular events. Given the potential discrepancies in comorbidity conditions among patients with prior cardiovascular events and those without, all the analyses were stratified by patients’ prior cardiovascular event status. We found the effects were not different among patients with prior cardiovascular events and those without. Regardless of patients’ prior cardiovascular event status, patients with larger testosterone declines (≥3.12 nmol/L, 90th percentile) since the last visit were more likely to experience myocardial infarction. In conclusion, recent pronounced testosterone drop-offs may affect the risk of cardiovascular events among hypogonadal men. Future longitudinal studies are needed to confirm our exploratory study findings.
Introduction
Testosterone is the principal male sex hormone responsible for the maturation of sexual organs and secondary sexual characteristics. It is also an important metabolic hormone for maintaining the overall physiological function, including carbohydrate, protein, and lipid metabolism in men [Citation1]. Low testosterone level has been consistently associated with risk factors for cardiovascular disease such as insulin resistance, hyperlipidemia, and metabolic syndrome. Animal models have demonstrated an anti-atherogenic action of testosterone in males, whereas testosterone deficiency promotes the early stages of atherogenesis, the first step of coronary heart disease [Citation2,Citation3]. Studies have also shown that low testosterone levels were associated with higher carotid intima media thickness [Citation4–6], which is a surrogate marker of atherosclerosis and a strong predictor of future clinical ischemic cardiac and cerebrovascular events [Citation7–9]. In a prospective cohort study of 3443 community-dwelling men seventy years and older, Yeap et al. reported that testosterone in the lowest quartile (<11.7 nmol/L) was associated with an increased stroke or transient ischemic attack incidence (hazard ratio (HR)=1.69, 95%CI: 1.15, 2.48) [Citation10].
In addition to the absolute testosterone level, remarkable changes in testosterone level may have an acute effect on disease development and progression. In animal studies, wild-type mice that went through castration experienced a pronounced testosterone decline within hours. Sudden testosterone declines induced macrophage infiltration and elevated inflammation biomarkers such as interleukin-6 (IL-6) and interleukin-1 beta (IL-1β), and lipid profiles such as cholesterol and triglyceride remarkably increased in the castrated mice as compared to the sham-operated mice. As a result, the castrated mice manifested exacerbated aortic aneurysm, a pathological phenotype of vascular aging, five weeks after castration [Citation11]. In human studies, St Sauver et al. found faster declines in testosterone level was associated with faster increases in prostate volume and lower urinary tract symptoms, and faster decreases in maximum flow rate [Citation12]. Our recent study [Citation13] investigating the dynamic patterns of testosterone on prostate cancer development found that hypogonadal men with a notable testosterone level decline during the study period had a higher risk of prostate cancer. Given the critical role that testosterone plays in maintaining the normal physiological function, it may also be important to know whether pronounced testosterone declines influence the risk of other adverse health outcomes such as cardiovascular disease, especially for those who already experienced testosterone deficiency that posed a baseline higher risk for many chronic conditions including cardiovascular disease. If notable testosterone declines are confirmed to be associated with an increased risk of cardiovascular disease, monitoring of testosterone levels might be necessary to deliver timely interventions when pronounced drop-offs of testosterone are detected in order to maintain a normal metabolic function and prevent cardiovascular events in men.
In this study, we used a clinical dataset of 376 hypogonadal men whose testosterone levels were below 12.1 nmol/L at study entry from a registry study in Germany, and conducted survival data analyses to investigate the effect of sudden testosterone declines on the risk of cardiovascular events. Given the potential discrepancies in comorbidity conditions among patients with prior cardiovascular events and those without, all the analyses were stratified by patients’ prior cardiovascular event status. Whether the effects of testosterone drop-offs on the risk of cardiovascular events were actually different among patients with prior cardiovascular events and those without was also examined.
Materials and methods
Study population
We used de-identified data from a registry study in Germany. Seven hundred and seventy-six hypogonadal men were recruited from one urology center in Bremerhaven, Germany from 2004 to 2016. Hypogonadism diagnosis was confirmed if they had total testosterone level ≤12.1 nmol/L and symptoms such as decreased libido, erectile dysfunction, depression, and fatigue, as assessed by the Aging Males’ Symptoms scale (AMS). 8 patients had primary hypogonadism according to patient history, all other patients had functional hypogonadism. The threshold of 12.1 nmol/L was selected based on clinical experience and confirmed by Bhasin et al. [Citation14]. Ethical guidelines formulated by the German Ärztekammer (German Medical Association) for observational studies in patients receiving standard treatment were followed. After receiving an explanation about the nature and the purpose of the study, all patients provided written consent to be included in the registry and have their data analyzed. Eligible participants without contraindications were given option of testosterone therapy at study entry. Four hundred of them decided to receive testosterone therapy, whereas three hundred and seventy-six opted against testosterone therapy for various reasons including the concern about the testosterone therapy safety issues. This study only used data from the participants who did not take treatment to avoid the influence of exogenous testosterone administration.
Testosterone measurement and cardiovascular outcome ascertainment
Participants were followed semi-annually for updates in serum testosterone levels. Lab measurement methods have been described elsewhere [Citation15]. Briefly, Alinity i-Module for the Abbott that performs chemiluminescent microparticle immunoassay (CMIA) was applied. The used kit is the 2nd generation testosterone reagent kit which has an intra assay variation of 2.8% and an inter assay variation of 3.1%. Cardiovascular events (i.e. myocardial infarction and stroke) occurring during follow-up were recorded. Cardiovascular events were partly reported in the form of “physician letters” from the hospital or the cardiologist/neurologist/family physician, and partly by patients themselves or relatives. The latter usually occurred when already scheduled patient visits had to be postponed due to an event.
Statistical analysis
In the descriptive analysis, characteristics were compared in the patients who experienced cardiovascular events during the follow-up period and those who did not.
A time-varying “testosterone drop” variable that is equal to the testosterone level measured at the last visit minus the testosterone level measured at the current visit was created. A positive value indicates the testosterone level declines since the last visit; a negative value indicates the testosterone level increases since the last visit. The larger the value of this variable, the larger change (drop/decline) this patient experienced between two adjacent visits.
Cox proportional hazards regression models were fitted to the data to investigate the association between testosterone drop since the last visit and the risk of cardiovascular events. Covariates that are closely related to the risk of cardiovascular risk including age at study entry, baseline smoking/drinking status, baseline comorbidities (type 2 diabetes, hypertension, dyslipidemia), and family history of coronary heart disease were included in the model. Though there has been a recent change in the definition of dyslipidemia considering the impact of gender on high-density lipoprotein (HDL) and the use of medication, by the time of this study, we defined dyslipidemia as HDL <40 mg/dL (1.03 mmol/L) or triglycerides ≥150 mg/dL (1.7 mmol/L), according to the previous harmonized definition of the metabolic syndrome [Citation16]. HR describing the risk of cardiovascular events and its 95% confidence interval (CI) was presented comparing (1) observations differing in 1 nmol/L testosterone drop; (2) observations of which testosterone drop was greater than or equal to the median to those of which testosterone drop was smaller than the median; (3) observations of which testosterone drop was greater than or equal to 75th percentile to those of which testosterone drop was smaller than 75th percentile; (4) observations of which testosterone drop was greater than or equal to 90th percentile to those of which testosterone drop was smaller than 90th percentile. We considered both negative and positive values of the testosterone drop variable for all the participants and all the visits when calculating the percentiles.
Analyses were stratified by participants’ prior cardiovascular event status; those with prior cardiovascular events and those without prior cardiovascular events. The significance of the interaction between testosterone drop since the last visit and patients’ prior cardiovascular event status was also tested to examine whether the effects of testosterone drop since the last visit were actually different in the two strata. Outcome variables were myocardial infarction, stroke, or any cardiovascular event (either myocardial infarction or stroke). Mean testosterone levels at each visit for patients who experience cardiovascular events during the study period and those who did not were presented separately. Testosterone changes over time for selected participants in each group were also depicted.
All analyses were performed with Stata/MP 14.0.
Results
As shown in , the baseline characteristics were in general different in the two groups.
Table 1. Characteristics of participants, by outcome status.
As compared to the participants who did not experience cardiovascular events, those who had cardiovascular events during the study period had a higher BMI, were more likely to be a smoker and alcohol user, have hypertension, diabetes, family history of coronary heart disease, and prior cardiovascular events, and had a longer follow-up period (p-value < 0.05). There was no difference in baseline age, testosterone levels, and the proportions of patients with dyslipidemia in the two groups (p-value > 0.05).
presents the Cox regression analysis results, stratified by patients’ prior cardiovascular event status. After adjusting for age at study entry, BMI, smoking and drinking status, family history of coronary heart disease, and baseline comorbidity (hypertension, diabetes, dyslipidemia), participants who experienced greater testosterone drop-offs in general had a higher risk of cardiovascular events, with HRs ranging from 0.95 to 3.01. For participants with prior cardiovascular events, those whose testosterone drop since the last visit was greater or equal to 3.12 nmol/L (90th percentile) had a 2.72 (95%CI: 1.37, 5.41) times higher risk of any cardiovascular event and 3.01 (95%CI: 1.22, 7.40) times higher risk of stroke. For other HRs, although a higher risk of cardiovascular events was observed in patients with larger testosterone drop-offs, those ratios were not different from 1 (p-value > 0.05).
Table 2. Cox proportional hazards regression of cardiovascular events on testosterone drop, after adjustment, by prior cardiovascular event status.
Though the stratum-specific HRs were slightly different among patients with prior cardiovascular events and those without, the interactions between testosterone declines since the last visit and patients’ prior cardiovascular event status were not different from 0 (p > 0.05, results not shown), indicating the effects of testosterone drop on the risk of cardiovascular events were not different in the two strata, based on formal statistical tests. Thus, a collapsed table describing this association, regardless of the patients’ prior cardiovascular event status was presented.
As shown in , patients whose testosterone decline since the last visit was greater or equal to 3.12 nmol/L (90th percentile) had a 1.93 (95%CI: 1.19, 3.11) times higher risk of any cardiovascular event and 2.09 (95%CI: 1.14, 3.86) times higher risk of myocardial infarction, after adjustment. A potential dose-response relationship between testosterone decline since the last visit and risk of cardiovascular events was observed; patients with higher testosterone declines since the last visit had a higher risk of cardiovascular events. This result matched our post hoc analysis results using stratified proportional hazards models (results not shown).
Table 3. Cox proportional hazards regression of cardiovascular events on testosterone drop, after adjustment.
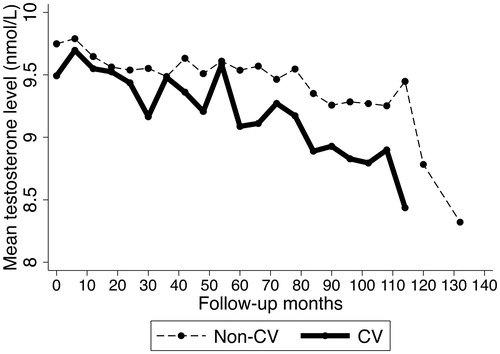
depicts the mean testosterone levels during the follow-up period in the patients who experienced cardiovascular events and those who did not. Both cardiovascular cases and non-cases had decreasing testosterone levels during the study period, as no exogenous testosterone was administrated to these patients. As compared to patients who did not have cardiovascular events, those who developed events had more variable changes in testosterone levels and overall “steeper” slope for the decreasing trend. There were also notable drop-offs observed around month 30, 60, and 110 for cases, and post 110 months for non-cases. Data on later follow-up periods beyond 110 months might be less precise due to the limited number of observations (n = 18).
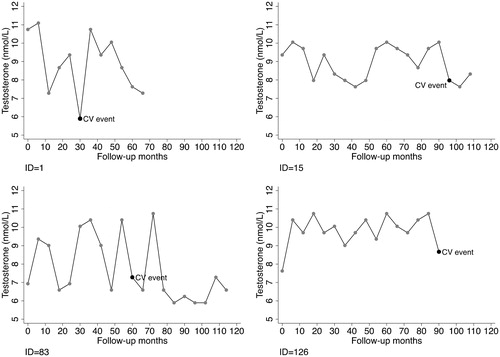
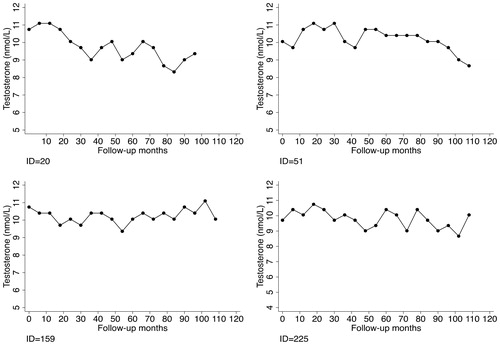
depicts the testosterone changes over time for selected patients who experienced cardiovascular events during the study period. They all experienced pronounced testosterone drop-offs before the event. For comparison, shows the testosterone changes for selected patients who did not experience cardiovascular events during the study period. Though the overall trend was decreasing, they did not have remarkable testosterone declines.
Discussion
In this cohort study, we explored the association between recent testosterone drop-offs and the risk of cardiovascular events, stratified by patients’ prior cardiovascular event status. We found the effects of testosterone declines since the last visit on the risk of cardiovascular events were not different among patients with prior cardiovascular events and those without, based on formal statistical tests. Regardless of patients’ prior cardiovascular event status, patients with larger testosterone declines (≥3.12 nmol/L, 90th percentile) since the last visit were more likely to experience myocardial infarction, but not stroke.
In contrast to women, who have a sudden cessation of gonadal function and pronounced sex hormone changes around menopause [Citation17], men typically experience a slow reduction in male hypothalamic-pituitary-gonadal axis function during middle to late adulthood, which results in a mild and gradual decline in testosterone levels through both central (hypothalamic-pituitary level, alterations in gonadotropin secretion) and peripheral (gonadal level, failure of Leydig cell function in the testicle) mechanisms [Citation18]. On average, this change is small, and the levels remain within the normal range in most men; approximately 25% of men experience a progressive decline and are diagnosed of testosterone deficiency, or hypogonadism during their 60s or 70s [Citation19], a condition of suppressed circulating testosterone levels and symptoms such as erectile dysfunction and decreased libido. In addition to poor physical performance and increased frailty, low testosterone level has been associated with obesity, greater waist circumference, diabetes, hypertension, and lower level of HDL cholesterol, which are strong predictors of future cardiovascular events [Citation19,Citation20]. Hypogonadism is common in men with metabolic syndrome and associated with poor glucose and lipid control, which are well-established risk factors for cardiovascular disease [Citation21]. In our previous study, we noticed increased HDL level as well as decreased total cholesterol, low-density lipoprotein (LDL), and triglyceride levels in the treatment group, while the levels increased in the control group except for HDL, which decreased over time. Other studies reported the effect of testosterone level on the functionality of HDL [Citation22,Citation23]. Additionally, researchers found testosterone deficiency was associated with premature coronary artery disease in men age ≤45 years and higher carotid intimal thickness in middle-aged diabetic men [Citation24,Citation25]. Atherosclerotic plaques and endothelial dysfunction were more likely to be found in patients with low testosterone [Citation19]. Additionally, testosterone deficiency has been associated with hepatic dysfunction and elevated liver enzyme levels that are associated with vascular inflammation reflecting rupture-prone vulnerable atherosclerotic plaques [Citation26] and impaired coronary flow reserve [Citation27].
Among patients who already have lower than normal testosterone levels, remarkable drop-offs may exacerbate the preexisting comorbidities and contribute to additional risk for subsequent cardiovascular events. Studies have shown that androgen-deprivation therapy (ADT), which aims to lower testosterone levels to castration levels using chemical (e.g. gonadotropin-releasing hormone (GnRH) antagonists) or surgical methods (bilateral orchiectomy) among advanced prostate cancer patients, causes a rapid decrease in testosterone and leads to detrimental cardiovascular adverse effects [Citation28]. The sudden testosterone declines resulting from ADT have been associated with an increase in fat mass, lipid panel, glycosylated hemoglobin levels, and insulin resistance within the initial months of treatment [Citation29,Citation30]. The metabolic changes that create an atherogenic risk following ADT might be due to sudden loss of androgen-mediated inhabitation of stem cell differentiation into adipocytes and an increase of pro-inflammatory cytokines [Citation28,Citation31]. Additionally, ADT-associated QT prolongation and changes in cardiomyocyte contractility may also contribute to an increased cardiovascular risk [Citation32,Citation33]. Many of these changes and alterations were observed to be greater in older individuals, who may have already experienced testosterone declines and shown decreased compliance to changes. This is consistent with our study findings that among hypogonadal men with low testosterone levels, sudden testosterone drop-offs were associated with an additional risk of cardiovascular events.
As compared to men without prior cardiovascular events, those with prior cardiovascular history might be more vulnerable to cardiovascular events if exposed to risk factors, given the pathological changes of their blood vessels and overall worse health conditions. However, in our study, we did not find any statistical difference in the effects of testosterone drop-offs on the risk of cardiovascular events among patients with prior cardiovascular events and those without. Previous studies that examined the association between the absolute testosterone level and the risk of cardiovascular events reported that there was no such association if their study participants were restricted to patients without prior cardiovascular events, and a positive association if their study participants were those with prior cardiovascular events [Citation34–36]. However, the authors used different source populations, and therefore alternative explanations might account for the difference in their study findings other than patients’ prior cardiovascular event status. In our study, we initially intended to investigate whether patients with prior cardiovascular history had a higher risk of cardiovascular events when experiencing notable testosterone declines as compared to those without, in order to provide evidence for future targeted intervention in the more susceptible population. However, due to the limited sample size in the stratified analyses, we were underpowered to detect the true difference if any. Future studies are needed to verify our study findings.
Notable drop-offs or large variations of testosterone levels not only increase the risk of cardiovascular events, but also relate to the development of other diseases. Researchers reported Parkinson’s Disease-related nigrostriatal pathologies in young male mice induced by castration, a surgical or chemical procedure that removes testicles that leads to sudden testosterone drops. These pathologies were normalized and the locomotor activities got improved after testosterone supplementation [Citation37]. In our recent study that investigated the dynamic patterns of testosterone level and risk of prostate cancer using the same data source, we found that among hypogonadal men, further declines or large variations of testosterone level over the study period were associated with an increased risk of prostate cancer, and those that had a younger age at hypogonadism diagnosis were at a higher risk of developing prostate cancer [Citation13]. In the current study, we also performed a secondary analysis to determine whether testosterone drop-offs since the last visit has an impact on the risk of prostate cancer, and the findings show that the HR for prostate cancer associated with each 1 nmol/L testosterone drop since the last visit was 1.72 (95%CI: 1.35, 2.19). In another study that examined the association between family history of prostate cancer and testosterone level using nationally representative National Health and Nutrition Examination Survey (NHANES) data, we found men with a family history of prostate cancer experienced a more pronounced drop-off in testosterone levels between early to middle adulthood, which may explain in part the excess prostate cancer risk associated with family history of prostate cancer [Citation38]. Further investigation is needed before any firm conclusion can be made. If that is the case, regular monitoring of testosterone levels in men might be useful to detect abnormal drop-offs or variations and deliver timely interventions (e.g. testosterone therapy) to prevent adverse health outcomes such as prostate cancer or cardiovascular events.
To our knowledge, this study is the first to investigate the effect of recent testosterone drop-offs on the risk of cardiovascular events. It provides insights into the etiologic role of testosterone in the development of cardiovascular events. In addition to the absolute testosterone level, the abnormal decline of testosterone levels among patients who already had testosterone deficiency may pose an additional cardiovascular risk for them. However, limitations should be noted. First, the study population consisted of European men who consulted urologic problems in a private urology center in Germany, and therefore our results may not be generalized to men of other nationalities or ethnicities. In addition, because the study population already had low testosterone levels, our conclusion about the effect of testosterone drop-offs on the risk of cardiovascular events may only apply to hypogonadal men. Future rigorously designed prospective cohort studies in a general male population with a diverse nationality/ethnicity makeup and a wide range of testosterone levels are needed. Second, there are several types of strokes, among which ischemic stroke is more closely related to atherosclerosis and excess lipid accumulation that sudden testosterone declines may contribute to. However, in this dataset, we did not have information on the specific type of stroke that the patient had, and it could be the reason we did not find any association between testosterone drop-offs and stroke in this study. Third, the cut-off points to categorize testosterone drop-offs in the analyses were arbitrary, given the exploratory nature of this analysis. Future longitudinal studies are needed to confirm the study findings. Lastly, given the close relationship between testosterone and sex hormone binding globulin (SHBG), we should report the changes of SHBG over time and its association with cardiovascular risk. However, by the time of this study, we did not have such information available.
In conclusion, recent pronounced testosterone drop-offs may affect the risk of cardiovascular events among hypogonadal men. The effects of testosterone drop since the last visit were the same among patients with prior cardiovascular events and those without. Regular monitoring of testosterone levels in aging men might be useful to detect abnormal drop-offs or variations and deliver timely interventions to prevent adverse health outcomes such as cardiovascular events. Future longitudinal studies in male populations with different characteristics from ours (e.g. testosterone range, race/ethnicity, nationality) are needed to confirm our exploratory study findings.
Disclosure statement
Dr. Farid Saad is a senior consultant for Bayer AG, Berlin. Dr. Ahmad Haider has received research support, lecture honoraria and travel grants from Bayer AG. Karim Sultan Haider has received lecture honoraria and travel grants from Bayer AG.
References
- Traish AM, Haider A, Haider KS, et al. Long-term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism. J Cardiovasc Pharmacol Ther. 2017;22(5):414–433.
- Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47–R71.
- Bruck B, Brehme U, Gugel N, et al. Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits. ATVB. 1997;17(10):2192–2199.
- Vikan T, Johnsen SH, Schirmer H, et al. Endogenous testosterone and the prospective association with carotid atherosclerosis in men: the Tromsø study. Eur J Epidemiol. 2009;24(6):289–295.
- Soisson V, Brailly-Tabard S, Empana JP, et al. Low plasma testosterone and elevated carotid intima-media thickness: importance of low-grade inflammation in elderly men. Atherosclerosis. 2012;223(1):244–249.
- Kwon H, Lee D-G, Kang HC, et al. The relationship between testosterone, metabolic syndrome, and mean carotid intima-media thickness in aging men. Aging Male. 2014;17(4):211–215.
- Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–467.
- Nezu T, Hosomi N, Aoki S, et al. Carotid intima-media thickness for atherosclerosis. J Atheroscler Thromb. 2016;23(1):18–31.
- O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340(1):14–22.
- Yeap BB, Hyde Z, Almeida OP, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94(7):2353–2359.
- Son BK, Kojima T, Ogawa S, et al. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J Endocrinol. 2019;241(3):307–317.
- Sarma AV, St. Sauver JL, Jacobson DJ, et al. Racial differences in longitudinal changes in serum prostate-specific antigen levels: the Olmsted county study and the flint men’s health study. Urology. 2014;83(1):88–93.
- Xu X, Zhang X, Zhong Y, et al. Dynamic patterns of testosterone levels within Individuals and risk of prostate cancer among hypogonadal men: a longitudinal study. J Urol. 2018;199(2):465–473.
- Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the framingham heart study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011;96(8):2430–2439.
- Sultan K, Haider A, Doros G, et al. Design and Conduct of a Real-World Single-Center Registry Study on Testosterone Therapy of Men with Hypogonadism. Androg Clin Res Ther. 2021;2(1):1–17.
- Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–480.
- Huhtaniemi I, Forti G. Male late-onset hypogonadism: pathogenesis, diagnosis and treatment. Nat Rev Urol. 2011;8(6):335–344.
- Jiang M, Huhtaniemi I. Polymorphisms in androgen and estrogen receptor genes: effects on male aging. Exp Gerontol. 2004;39(11–12):1603–1611.
- Kloner RA, Carson C, Dobs A, et al. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545–557.
- Srinath R, Gottesman RF, Hill Golden S, et al. Association between endogenous testosterone and cerebrovascular disease in the ARIC study (Atherosclerosis Risk in Communities). Stroke. 2016;47(11):2682–2688.
- Laaksonen DE, Niskanen L, Punnonen K, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab. 2005;90(2):712–719.
- Adorni MP, Zimetti F, Cangiano B, et al. High-density lipoprotein function is reduced in patients affected by genetic or idiopathic hypogonadism. J Clin Endocrinol Metab. 2019;104(8):3097–3107.
- Sirtori CR, Ruscica M, Calabresi L, et al. HDL therapy today: from atherosclerosis, to stent compatibility to heart failure. Ann Med. 2019;51(7–8):345–359.
- Alkamel A, Shafiee A, Jalali A, et al. The association between premature coronary artery disease and level of testosterone in young adult males. Arch Iran Med. 2014;17(8):545–550. doi:014178/AIM.005
- Farias JM, Tinetti M, Khoury M, et al. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99(12):4698–4703.
- Choi KM, Han K, Park S, et al. Implication of liver enzymes on incident cardiovascular diseases and mortality: a nationwide population-based cohort study. Sci Rep. 2018;8(1):3764.
- Yilmaz Y, Kurt R, Yonal O, et al. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211(1):182–186.
- Gupta D, Lee Chuy K, Yang JC, et al. Cardiovascular and metabolic effects of androgen-deprivation therapy for prostate cancer. J Oncol Pract. 2018;14(10):580–587.
- Smith MR. Changes in body composition during hormonal therapy for prostate cancer. Clin Prostate Cancer. 2003;2(1):18–21.
- Tzortzis V, Samarinas M, Zachos I, et al. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones (Athens). 2017;16(2):115–123.
- Zareba P, Duivenvoorden W, Leong DP, et al. Androgen deprivation therapy and cardiovascular disease: what is the linking mechanism? Ther Adv Urol. 2016;8(2):118–129.
- Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–4456.
- Bosco C, Bosnyak Z, Malmberg A, et al. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68(3):386–396.
- Collet T-H, Ewing SK, Ensrud KE. Endogenous testosterone levels and the risk of incident cardiovascular events in elderly men: the MrOS prospective study. J Endocr Soc. 2020;4(5):1–15.
- Chmiel A, Mizia-Stec K, Wierzbicka-Chmiel J, et al. Low testosterone and sexual symptoms in men with acute coronary syndrome can be used to predict major adverse cardiovascular events during long-term follow-up. Andrology. 2015;3(6):1113–1118.
- Srinath R, Golden SH, Carson KA, et al. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2015;100(4):1602–1608.
- Khasnavis S, Ghosh A, Roy A, et al. Castration induces parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem. 2013;288(29):20843–20855.
- Zhang X, Zhong Y, Taylor N, et al. Family history of prostate cancer and age-related trend of testosterone levels among US males: NHANES 2003–2004. Andrology. 2019;7(3):288–292.