Abstract
Background
Testosterone is associated with sexual desire and performance in men, but little is known about cognitive mechanisms underlying this relationship. Even less is known about the influence of estradiol, despite its production from testosterone, and high receptor density in brain regions related to male sexual behavior.
Method
We used eye-tracking to compare men’s visual attention to images of fully clothed (i.e. neutral) and minimally clothed (i.e. sexy) models in three groups: androgen-deprived (n = 6) and not androgen-deprived with prostate cancer (n = 11), and healthy controls (n = 7). We also assessed effects of serum testosterone, estradiol, and sex hormone-binding globulin levels.
Results
We found no group effect for fixations to sexy compared to neutral images, and no influence of testosterone on either total fixations, or proportion of fixations to sexy images. In contrast, we found that sex hormone binding globulin positively predicted total fixations, and estradiol positively predicted proportion of total fixations on sexy images--regardless of androgen treatment status.
Conclusion
Our results suggest that visual attention to sexual stimuli in men may be significantly affected by hormones. This has potential implications for clinical populations that experience sexual side effects, such as prostate cancer patients on androgen deprivation therapy.
Introduction
Sexual arousal involves multidirectional relationships between physiological, psychological, and behavioral processes. Psychological processes, specifically, involve the evaluation of sexually relevant stimuli. These can be both involuntary (rapid, automatic, and unconscious) and voluntary (slower, conscious, and controlled) assessments [Citation1,Citation2]. Automatic assessments take place first, where stimuli are associated with pre-existing knowledge (i.e. meaning), and physiological (e.g. genital) responses are initiated. Controlled assessments come afterward, where attention is directed towards the stimuli, and relevant emotions are experienced. At that point, individuals have a subjective sense of whether they are sexually aroused or not, and become aware of both the stimulus and their physiological response to it. Approach (or avoidance) behavior is then initiated, which facilitates (or hampers) reproduction. Steroid hormones, such as testosterone (T) and estradiol (E2), could be involved in any or all of these processes.
In men, T production is centrally regulated through the secretion of gonadotropic releasing hormone (GnRH) from the hypothalamus. This stimulates the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland into the bloodstream. Luteinizing hormone then facilitates the production of T from the testes, which subsequently signals back to the hypothalamus to slow GnRH release (i.e. negative feedback). Men’s E2, on the other hand, is produced mainly from the conversion of T via the enzyme aromatase. T and E2 levels are interdependent, however. Both T and E2 can regulate the hypothalamic-pituitary-gonadal axis via negative feedback and thus alter gonadal hormone production [Citation3]. Further, the actions of both steroids are influenced in part by circulating levels of sex hormone-binding globulin (SHBG), a liver-derived protein that binds T and E2, and transports these gonadal steroids in an inactive form in the serum.
It is not surprising that previous work has demonstrated a role for both T and E2 in maintaining sexual function in both eugonadal and hypogonadal men. Compared to E2, however, T appears to be the stronger regulator of sexual interest in males. Indeed, Bagatell et al. [Citation4] showed that compared to T replacement alone, T replacement plus an aromatase inhibitor (i.e. blocking the conversion of T to E2) had no impact on sexual interest or activity in healthy men rendered hypogonadal with a GnRH agonist. Indeed, although positive effects of supplemental E2 on sexual behavior (e.g. mounting, intromissions) in castrated rodents are well-documented, results from the few studies with eugonadal men have been equivocal [reviewed in Citation5].
In contrast, in a cohort of men who were treated with a GnRH agonist to suppress T and E2, Finkelstein et al. [Citation6] found that those who received T replacement plus an aromatase inhibitor experienced greater reductions in sexual desire than those who received T replacement alone. Specifically, the authors found a dose-dependent effect where men with higher serum T(e.g. >200 ng/dL) and E2 (≥10 pg/mL) reported smaller changes in sexual desire than those with lower E2 (<10 pg/mL).These results suggest that in combination with T, E2 plays a role in men’s sexual interest. However, the relative contributions of T and E2 have not been well studied. Specifically, the ways in which either hormone influences the cognitive processing of sexual stimuli prior to the subjective experience of sexual interest (e.g. attention to sexual stimuli) are unclear.
Using eye-tracking technology, researchers can investigate automatic cognitive responses to visual sexual stimuli. Such measures are simple to use and non-invasive. Eye-tracking can be used to quantify not only what participants look at in a visual image (i.e. visual attention and number of fixations), but how long they look at it for (i.e. duration of fixation), and how interested they are in it via pupil dilation [Citation7,Citation8]. Indeed, several eye-tracking studies have demonstrated that healthy heterosexual men look quickly and preferentially at nude compared to fully clothed women’s bodies [Citation8–10]. They also look preferentially at regions of the body associated with women’s health and fertility, such as the face, breasts, and waist [Citation10–12]. Visual fixation on these areas is also positively associated with physiological measures of sexual interest, including pupil dilation [Citation10] and increase in penile circumference [Citation13].
Huberman, Maracle, & Chivers [Citation14] found that heterosexual men’s self-reported attention to content mediated the positive relationship between their genital responses and self-reported arousal in response to sexually explicit videos. The same mediation effect was not observed for women. Although hormone levels were not assessed in this study, this could suggest that T is the dominant hormone that elicits visual attention to relevant stimuli in men. Only one study [Citation15] has explicitly examined the effects of T on visual attention to sexual stimuli in healthy eugonadal men using eye-tracking. That study showed that T positively predicts the duration of fixations to photographs of heterosexual couples engaged in sex, and that this relationship is strengthened for participants who report a higher frequency of viewing erotic material outside of the study. Given the reciprocal relationship between T levels and sexual activity in men [reviewed in Citation16], this latter result supports the hypothesis that T levels positively influence visual attention to sexually relevant stimuli.
No studies have investigated E2’s influence on cognitive processing of sexual stimuli in males, but several lines of evidence suggest a possible link. First, estrogen receptors ERα and ERβ are found in various brain centers associated with reproductive function, including the sexually dimorphic nucleus of the preoptic area (SN-POA) and the medial amygdala, both of which are involved in sexual arousal [Citation7]. Second, E2 levels have been positively associated with visual recognition and visual memory in both healthy young men [Citation17], and older men with prostate cancer (PCa) [Citation18]. Third, preclinical evidence shows that estrogen receptors are present in brain regions involved in visual processing, such as the superior colliculus [Citation19,Citation20] which is known to play a role in visual attention and fixation [Citation21]. Lastly, several studies of androgen-deprived men, who received E2 supplements as part of PCa treatment, retain some sexual interest and sexual activity, compared to men who are androgen-deprived, but did not receive E2 [Citation22].
Investigating the relationships between hormones, psychological, and physiological responses is important for understanding male sexuality in general but may be particularly important for men treated for PCa with androgen deprivation therapy (ADT). Erectile dysfunction and loss of sexual desire are common among these men, which often results in psychological discomfort and relationship stress [Citation22,Citation23]. Such sexual dysfunction is typically attributed to the men’s lack of T, but as noted above, ADT also reduces endogenous E2. Further, serum SHBG is negatively correlated with T and E2, higher in aged males [Citation24], and may exert its own effects on neural functions [Citation24,Citation25]. Thus, SHBG could also be an important factor in men’s processing of sexual stimuli. Here we report data from a study designed to directly investigate relationships between T, E2, SHBG, and visual attention to sexual stimuli in PCa patients either treated with ADT or not, as well as age-matched healthy controls.
Previously, we showed that men on ADT failed to demonstrate the same bias toward preferential fixation on sexy compared to non-sexy images shown by both men with prostate cancer who were not receiving ADT as well as healthy men without PCa [Citation26]. There we utilized an eye-tracking protocol where participants did not know their visual activity was being tracked. However, we did not measure participants’ hormone levels directly. Thus, we could not isolate any effect of T versus other hormones such as E2, on the eye-tracking data.
In the present study, we used the same eye-tracking protocol to further evaluate the effect of ADT on number of fixations to sexy (i.e. minimally-clothed) and non-sexy (fully-clothed) images of male and female runway models. To explore hormone mechanisms that might underlie this relationship, we also assessed the effects of baseline serum T, E2, and SHBG on number of fixations to the images. As before, we expected that men on ADT would exhibit fewer fixations to sexy and non-sexy images compared to the non-ADT and healthy control participants. In addition, we hypothesized that both T and E2 would positively predict number of fixations to sexy images.
Materials and methods
Patients/subjects
Twenty-nine men ranging in age from 56-84 years (68.34 ± 7.65 years) were recruited from PCa support groups and community centers in Greater Vancouver, British Columbia, Canada, to participate in this study. All were told we were conducting a study on “Visual Attention and Cognition” in older men, and that they would be required to provide a blood sample within 1-2 weeks of their participation date in order to assess their hormone levels. All participants were fluent in English and reported normal or corrected-to-normal vision. Twenty-eight participants (97%) were heterosexual and 25 (86%) were in committed, long-term relationships lasting between 2 and 50 years (27.68 ± 15.95 years).
We sought both hypogonadal and eugonadal men for the study in order to have a wide range of baseline steroid hormone concentrations among participants. Thus, our sample included men with PCa who were being treated with ADT (ADT group, n = 8), men with PCa who were not being treated with ADT (PC group, n = 13), and healthy men without PCa (HC group, n = 8). Patients with late-stage disease, metastatic presentation that could have interfered with their mobility or comfort while sitting, or any self-reported psychological, familial, sociological issues that would preclude them from complying with the study protocol were excluded from the study.
Images and eye-tracking
To test whether gonadal hormone concentrations influenced visual attention to sexual stimuli, we took advantage of a pre-existing protocol that has been used to identify social influences on looking behavior in undergraduates [Citation27,Citation28], as well as elderly men with and without PCa [Citation26]. We used a desktop eye-tracking system (RED, SMI, Teltow, Germany) to track eye movements while participants viewed images of male and female runway models on an integrated desktop computer monitor that inconspicuously housed external eye-tracking hardware along its bottom edge. Images were presented at a resolution of 1,680 × 1,050 pixels. Sampling rate was 120 Hz, tracking range was 40 × 20 cm at a 70 cm distance from the 56 cm (22 inch) monitor, accuracy was .4°, and spatial resolution was .03°.
Images consisted of 20 same-sex pairs of colored photos of runway models (10 male and 10 female). Each pair was facing forward, with their entire body visible. One photo depicted a model minimally clothed (MC) (e.g. wearing a bathing suit) and one photo depicted a model fully clothed (FC) (). Image pairs were presented sequentially for 10 s each, in randomized same-sex pairs, for all participants. Image pairs bordered central fixation by 4.2° (left-right separation between images = 8.4°). Whether FC and MC images were shown on the left or right sides of the screen was counterbalanced. We recorded the total number of fixations on each of the FC and MC images for each participant.
Given that the images were all of runway models, we assumed that they would be considered physically attractive to participants. Models of both sexes were included in the image pairs as per the pre-existing study protocol. Although most of our participants were heterosexual, inclusion of both male and female models allowed us to assess whether any observed differences in viewing behavior were due to the sex of the visual targets. It is important to note, however, we found no effect of model sex on fixations on the images in our previous study [Citation26].
Psychological and health measures
Because some of our participants were being treated for PCa, we needed to ensure that their visual attention was not influenced by any clinically significant comorbidities, levels of depression, or treatment side effects. Thus, all participants completed the following questionnaires:
Arizona sexual experiences scale (ASEX)
A 5-item questionnaire assesses general sexual functioning (e.g. sexual desire, sexual arousal, ease of obtaining an erection, ease of reaching an orgasm, orgasm satisfaction) [Citation29]. Higher scores indicate greater sexual dysfunction.
Expanded prostate cancer index composite (EPIC)
An overall quality of life measure that assesses symptoms and associated bother resulting from PCa treatment (e.g. bladder and bowel incontinence, impotence, gynecomastia) [Citation30]. The full measure includes urinary, bowel, sexual, and hormonal domains; however, we only included the sexual function (EPIC-SF), sexual bother (EPIC-SB), hormone function (EPIC-HF), and hormone bother (EPIC-HB) subscales because previous work showed no effect of urinary and bowel domains on viewing behavior [Citation22]. Lower scores indicate greater dysfunction and greater bother.
Center for epidemiologic studies depression scale (CES-D)
A 20-item assessment of depressive symptoms [Citation31]. Higher scores indicate greater depression.
Attitudes about men scale (AAMS)
A 5-item scale that measures traditional attitudes regarding male gender roles (e.g. risk-taking is more appropriate for men than women, men should be self-sufficient and not ask for help, men should be physically strong and tough) [Citation32]. We included this measure to ensure that viewing behavior was not influenced by attitudes regarding masculinity and heterosexuality. Higher scores indicate a stronger belief in traditional masculinity and male behavior.
Comfort with sexual imagery
In order to ensure that any visual responses to the images we used were not due to surprise, novelty, or embarrassment, we assessed participants’ comfort with potentially provocative sexual images. We asked two questions as part of the demographic questionnaire. These were: “How often do you purposefully view erotica?” and “How comfortable are you with erotica?” In response to the first question, participants selected one of seven choices: never, 1–2 times a month, at least once a week, at least twice a week, about every two days, almost every day, or every day. For data analysis, we quantified these as ranging from 1 (never) to 7 (every day). In response to the second question, participants select the number that best applied to their level of comfort on a 7-point Likert scale ranging from 1 (very uncomfortable) to 7 (extremely comfortable).
Serum hormone concentrations
Participants provided blood samples through local government laboratory facilities (LifeLabs, http://www.lifelabs.com). They were permitted to visit any of the 40+ facility locations in the area, at their convenience. Blood samples were assayed for T, E2, and SHBG according to standard facility protocol. Median time between participation in the eye-tracking protocol and blood collection was 5 days.
Procedure
Following written informed consent, participants were told that they would be viewing images on a computer screen. They were then told that the types of images they would view would be randomly determined by them choosing a slip of paper from a cup. They were told that categories included household goods, motor vehicles, runway models, etc. Unbeknownst to the participant, all slips of paper in the cup contained the same image type—runway models. Following image category selection, participants were led to a separate room containing a chair, desk, and computer outfitted with the eye-tracking system. It was crucial that participants were unaware that their gaze was being monitored to prevent them from biasing their natural viewing behavior in ways they might believe to be more socially acceptable (e.g. away from or less often at sexual images) [Citation27,Citation28].
Once seated at the desk, participants were asked to view a colored circle on the monitor and told the activity was part of an Ishihara color blindness test [Citation33]. In actuality, the Ishihara images were used to calibrate the participants’ eye movements and gaze fixations for the eye tracker. Specifically, participants were instructed to follow with their eyes a colored circle that moved through 9 locations on the monitor, then report any change in color or shape of the circle. Once calibration was completed, participants were told that they would next view the images they had selected from the cup. Each participant was instructed to look at the images presented on the screen as they might normally do. No additional instructions were provided. The experimenter then left the room so that the participants took the visual attention tests in isolation.
After completing the eye-tracking task, participants completed the psychological and health questionnaires. They were then fully debriefed, informed of the true nature of the study, and given an opportunity to ask questions. None of the participants was aware of the actual study hypothesis before their eye-tracking data were collected. Participants received $20 to cover transportation and parking costs. All study procedures were reviewed and approved by the University of British Columbia Clinical Research Ethics Board (Approval #H15-01993) and the Trinity Western University Human Research Ethics Board (Approval #16ED06).
Data analysis
We assessed group differences in comfort and experience with sexual imagery, psychological and health variables, numbers of fixations on MC and FC images, and serum T, E2, and SHBG by conducting a series of one-way ANOVAs. LSD comparisons were used to follow up significant effects. We conducted a mixed (1 between, 2 within) model ANOVA to test for the effects of group, image type (MC, FC), model’s sex (male, female), and their interactions on number of fixations. Subsequent mixed (1 between, 1 within) ANCOVAs were run to test effects of group, image type, and sexual dysfunction, and their interactions on number of fixations. We then used multiple linear regression to test for effects of hormones and ADT treatment on total number of fixations, and proportion of total fixations on MC images. All variables were standardized prior to regression analyses.
Results
Data from 5 participants (2 HC, 1 PC, 2 ADT) were excluded due to insufficient eye movement (≤200 fixations across all images) during the experiment. In addition, because participants were not aware that their eye movements were being recorded while viewing the images, shifts in their body position caused their overall eye movement pattern to move slightly away from the image boundaries for some image pairs. Such shifts were detected in data from an additional 3 participants (2 HC, 1 PC). Total fixation numbers for these participants were corrected by excluding fixation counts for affected images, taking the average number of fixations per remaining image, and multiplying that number by 20 (i.e. the total number of images).
Thus, our final sample consisted of 24 men (6 ADT, 11 PC, and 7 HC groups). All except 1 (PCa group) reported being heterosexual, and all but 4 (1 ADT, 2 PC, 1 HC) reported being in long-term romantic relationships for 29.50 ± 14.80 years.
Comfort with sexual imagery
We found a significant difference between groups in reported frequency of viewing erotic material (F(2,21) = 7.10, p = .004, η2(partial) = 0.40) where men in the HC group (3.00 ± 0.82) reported viewing erotica significantly more often than men in the PC (1.73 ± 1.19, p = .014) and ADT (1.00 ± 0.63, p = .001) groups. The difference between the PC and ADT groups was not significant (p = .159). In contrast, there was no significant difference between groups in reported comfort with erotica (F(2,21) = 0.87, p = .432, η2(partial) = 0.08).
Psychological and health characteristics
Descriptive statistics for participant scores on all psychological and health measures are shown in .
Table 1. Descriptive statistics for psychological and health variables.
Sexual function
As expected, we found evidence for sexual dysfunction in the ADT and PC groups. First, there was a significant group difference on the total ASEX score (F(2,21) = 12.55, p <.001, η2(partial) = 0.54). Men in the ADT group scored significantly higher than men in the PC (p = .002) and HC (p <.001) groups, indicating more sexual dysfunction. The difference between the PC and HC groups was not significant (p = .077). Second, we found a significant group difference for EPIC-SF scores (F(2,19) = 12.16, p <.001, η2(partial) = 0.56), where men in the ADT group scored significantly lower (i.e. greater dysfunction) than men in the PC (p = .007) and HC (p <.001) groups. Men in the PC group also scored significantly lower than men in the HC group (p = .015), reflecting some sexual dysfunction in PC participants. Scores on these measures were highly correlated with each other (r = −0.93, p <.001), suggesting that participants’ sexual dysfunction was reliably measured by both scales. The EPIC-SB scores did not differ significantly among groups (F(2,19) = 2.72, p = .091, η2(partial) = 0.22).
Hormone function
Also as expected, we found evidence of hormone dysfunction and hormone bother in the ADT group compared to the PC and HC groups. There was a significant group difference on the EPIC-HF (F(2,21) = 5.29, p = .014, η2(partial) = 0.34), where men in the ADT group scored significantly lower (i.e. greater dysfunction) than men in the PC (p = .034) and HC (p = .004) groups. In contrast, there was no significant difference between scores from men in the PC and HC groups (p = .201).
There was also a group difference on the EPIC-HB (F(2,21) = 6.38, p = .007, η2(partial) = 0.38). Men in the ADT group scored significantly lower than men in the PC (p = .011) and HC (p = .002) groups, indicating greater self-reported distress from hormone dysfunction. In contrast, there was no significant difference between scores from men in the PC and HC groups (p = .312).
Depressive symptoms
We found no evidence of group differences in self-reported symptoms of depression. There was no significant difference between men’s scores on the CES-D (F(2,21) = 1.06, p = .363, η2(partial) = 0.09), a measure of their current mood state.
Adherence to masculine gender roles
We also found no evidence that men in our study differed in their adherence to traditional views of men’s gender roles or masculinity. There was no significant difference in AAMS scores between groups (F(2,21) = 0.05, p =.953, η2(partial) <0.01).
Viewing behavior
Total number of fixations on the sexy and neutral images across groups are shown in . As expected, we found a main effect of image type (F(1,21) = 18.92, p <.001, η2(partial) = 0.47), with participants fixating more on MC (360.88 ± 90.98) compared to FC (260.25 ± 66.12) images. Contrary to our predictions, however, neither the main effect of group (F(2,21) = 1.74, p =.200, η2(partial) = 0.14), or the interaction between image type and group (F(2,21) = .08, p = .924, η2(partial) <.01) were significant, suggesting that ADT did not exclusively influence visual attention to the images. There was also no significant effect of model’s sex (F(1,21) = 1.19, p = .288, η2(partial) <0.01, or any other significant 2- or 3-way interactions (all ps ≥.524).
Table 2. Total number of fixations on sexy and neutral images across treatment groups.
In order to explore any effect of sexual dysfunction on this pattern of results, we also ran a mixed model ANOVA with image type, group, and their interaction with ASEX score included as a covariate. The main effect of image type was no longer significant (F(1,20) = 2.66, p = .119, η2(partial) = 0.12), and again, neither the main effect of group (F(2,20) = 2.86, p = .081, η2(partial) = 0.22) nor image type by group interaction (F(2,20) = 0.28, p = .758, η2(partial) = 0.03) was significant. Similar analyses including frequency of viewing erotic material showed the same pattern of results. Neither the main effects of image type, group, or their interaction were significant (F(1,20) = 1.90, p = .183, η2(partial) = 0.09, F(2,20) = 1.54, p = .239, η2(partial) = 0.13, and F(2,20) = 0.18, p = 0.833, η2(partial) = 0.02, respectively).
Hormone effects
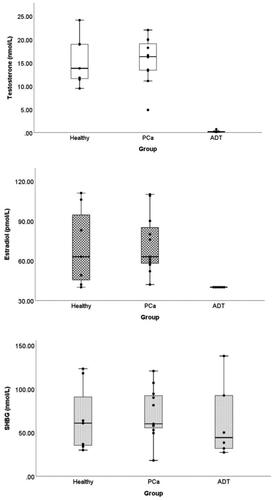
Seven participants (6 ADT, 1 HC) had undetectable concentrations of E2 (<40 pmol/L). Those participants were assigned a value of 40 pmol/L, the lowest detection limit for the assay, for all further analyses. Distributions of individual hormone values, by group, are shown in . As expected, ADT participants had significantly lower levels of T and E2, but not SHBG, than the PC and HC groups ().
Table 3. Serum hormone concentrations across treatment groups.
To determine the independent effects of each hormone on visual attention to sexual images, we ran linear regressions with total T, E2, SHBG, and ADT (0 = No, 1 = Yes) as predictor variables and total number of fixations, and proportion of total fixations on MC images as the dependent variables. All variables (except ADT) were standardized prior to analysis.
Total number of fixations across image types
Regression results for total fixations are shown in . Unexpectedly, we found that SHBG was a significant predictor of total fixations whereas E2, T, and ADT status were not. Adding frequency of viewing erotic material to the model did not change this pattern of results; however, after adding ASEX scores to the model, both SHBG and ADT were significant positive predictors, and ASEX was a significant negative predictor, of total fixations. These results are also shown in .
Table 4. Regression results for total number of fixations.
We then ran these analyses with free T rather than total T. Although there was a marginally significant negative correlation between free T and total number of fixations (r = −0.39, p = .059), replacing total T with free T did not change the pattern of results above, with or without the inclusion of frequency of viewing erotic material or ASEX in the model. Free T was not a significant predictor of total number of fixations (semi-partial r = 0.10, p = .584; with frequency of viewing erotic material: semi-partial r = 0.004, p = .979; with ASEX: semi-partial r = 0.01, p = .973).
Proportion of fixations on minimally clothed images
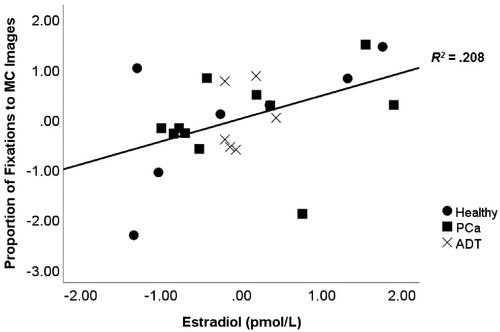
Regression results for proportion of fixations on MC images are shown in . Here, we found that E2 was a significant positive predictor, whereas T, SHBG, and ADT were not. The partial correlation between E2 and proportion of fixations on MC images is shown in . Importantly, adding either ASEX or frequency of viewing erotic materials to the model did not change this pattern of results—E2 remained a significant predictor whereas effects of T, SHBG, ADT, and ASEX were not significant ().
Table 5. Regression results for proportion of total fixations on MC images.
We then ran these analyses with free T rather than total T. Free T was not significantly correlated with proportion of fixations on MC images (r = 0.02, p = .935). Further, the pattern of results obtained in the regressions remained the same. Free T was not a significant predictor of proportion of fixations to MC images regardless of whether frequency of viewing erotic material or ASEX scores were included in the model (semi-partial r = −0.15, p = .473; with frequency of viewing erotic material: semi-partial r = −0.18, p = .386; with ASEX: semi-partial r = −0.16, p = .436).
Discussion
Although we emphasize that our results should be interpreted with caution due to small sample size, we provide here novel evidence that hormones facilitate visual attention in elderly men. In particular, our findings suggest that baseline concentrations of E2 and SHBG may participate in men’s ability to selectively attend to sexually relevant, as opposed to all types, of visual material. Contrary to our predictions, we did not find an exclusive effect of androgen deprivation on fixations to sexy compared to non-sexy images, or an association with T. However, we did find a positive association between attention to sexual images (i.e. minimally clothed models) and E2. These results align with those of previous studies that showed E2 had a protective effect on sexual desire in young eugonadal men [Citation6] as well as sexual activity in men with PCa [Citation34]. In addition, we unexpectedly found that although free T levels were marginally negatively correlated with attention to all images, SHBG exerts its own, stronger influence on visual attention, such that those with higher levels of SHBG fixated more on all image types.
Hormonal influences on visual attention to sexual stimuli
Importantly, our preliminary finding that E2 positively predicts visual attention to sexual images in older men suggests that, in the context of male sexual behavior, E2 may influence early, rapid, and involuntary attentional responses to relevant stimuli. Although the precise neurobiological mechanisms for this effect remain unclear, one possibility is that E2 works by activating estrogen receptors in brain regions that process visual information. In rodents, estrogen receptors are present in brain regions involved in visual processing, such as the superior colliculus [Citation19,Citation20]. In addition, the superior colliculus is known to play a role in visual attention and fixation [Citation21]. Considering that various brain centers for male sexual behavior (e.g. preoptic area, medial amygdala, bed nucleus of the stria terminalis) are rich in estrogen receptors [Citation35], there may be a potential connection (direct or indirect) between these regions and brain centers that process visual input. However, we are unaware of any studies that have documented such connectivity in humans, leaving this area open for future investigation.
Unexpectedly, and unlike previous work [e.g. 4,15] we failed to find a clear association between T and visual attention. One possible explanation for this finding is that T is associated with later, more voluntary, controlled visual processing. Indeed, Rupp and Wallen [Citation15] showed that T was associated with duration of fixations to sexual images, and that this relationship was stronger in men who viewed erotic material more often. Individuals with higher T in that study may have exhibited higher levels of sexual interest, thus looking longer than those with lower T at sexual images. Similarly, at least one study showed that T facilitated selective (i.e. voluntary) attention to relevant auditory stimuli when individuals were presented with sexual distractors (i.e. voluntary control of attention) [Citation36]. In contrast, E2 may work to orient attention toward relevant sexual images prior to any conscious awareness of their content or level of interest. To our knowledge, these hypotheses have not been directly tested.
Another possibility is that T facilitates sexual desire through its aromatization to E2 [see Citation37]. Indeed, T and E2 were moderately correlated in the present study (r = 0.535, p = .007), so previous work on T [e.g. 15], which had not simultaneously controlled for E2 levels, may have been detecting effects, at least in part, due to E2 rather than T exclusively. This line of research also remains open for further study.
Our finding that SHBG may influence visual attention more generally also warrants further investigation. Although SHBG increases with age in both men and women, its exclusive effects on cognitive processing are not well studied. Since men with higher levels of SHBG fixated more on all image types in our study, this could indicate that SHBG disrupts involuntary attention to, or perception of, relevant visual stimuli. Indeed, several studies have demonstrated a positive link between SHBG and cognitive decline [e.g. Citation38,Citation39; but see Citation40,Citation41] in men and women. Men with higher levels of SHBG could simply be more attentive to all potentially relevant stimuli, lacking the ability to hone in on those of greatest importance. No studies have investigated this possibility, but there is some evidence suggesting increased sexual receptivity in response to SHBG infusion into the brains of female rodents [Citation24]. Further research on the role of SHBG in both visual attention and sexual cognition would help elucidate these hypotheses.
Sexual impact of prostate cancer treatment
Our results suggest that ADT use, together with low self-reported sexual dysfunction and high SHBG levels, predicts greater number of fixations to both sexy and non-sexy images (i.e. total fixations). This finding has direct implications to PCa patients, many of whom are on ADT, which suppresses their gonadal hormone titers to castrate levels and negatively affect their sexual function. Together with our previous research showing that men on ADT fail to exhibit the normal eugonadal bias toward sexy versus non-sexy images [Citation26], our current findings provide additional evidence that men on ADT may fail to fixate preferentially on sexually relevant visual stimuli under certain conditions. Lack of attention to sexually relevant stimuli for men may translate into decreased sexual interest, reduced sexual activity, and eroding sexual relationships. These are common adverse effects for ADT for PCa patients [Citation23].
Knowing about the potential relationships between ADT and sexual cognition can help clinicians treating PCa patients (and their partners) and better inform the psychological impacts of ADT. Well-informed patients will be better prepared to accept and manage the side effects of treatment. Such information can be integrated into education programs for patients, who are starting on ADT [e.g. Citation42,Citation43]. Wibowo et al. [Citation43] showed that patients benefit in terms of elevated self-efficacy for managing ADT side effects if they are informed of those adverse effects when starting treatment. Further, clinicians should consider testing for steroid hormone and SHBG levels in older men experiencing sexual dysfunction, since our data suggest that both influence attention to sexual stimuli regardless of androgen status.
Our study demonstrates that eye-tracking can be used to study hormonal mechanisms underlying visual attention to sexual stimuli, especially in clinical populations. Such technology is increasingly used in studies of visual attention ranging from product advertising research to documenting paraphilias in forensic assessments. Our study demonstrates that gonadal hormone profiles can influence patterns of gaze fixation. This suggests that future eye-tracking studies may yield stronger results if data on hormonal status are collected and controlled for.
Limitations
There are several limitations in our study. First, our sample size is small. Recruiting participants for this study was challenging given their ages, medical conditions, and motivation to participate. We also note that there may have been sampling bias, as patients, who are motivated in advancing PCa research, may exhibit behaviors that are less typical of the average PCa patient or average healthy community member. Thus, we emphasize that our results should be considered preliminary.
Second, we included several self-report questionnaires that asked for very personal information (e.g. erectile dysfunction, depression, cancer treatment history, comorbidities). Because these data were not confirmed by clinicians and may have been subject to social desirability bias, we cannot validate their accuracy. Similarly, we did not collect detailed data on existing physiological (e.g. heart disease) or psychological (e.g. anxiety, neurological conditions) comorbities, or their treatments, so we are unable to determine whether these may have influenced our results. Further although our results suggest that men on ADT might experience higher levels of sexual dysfunction than the other groups, they did not report lower levels of sexual bother. One possible reason for this is that they may have adapted to low sexual desire and thus their willingness to be involved in sexual activity is dampened too. Future studies looking at this relationship will be helpful in explaining this discrepancy.
Lastly, it is important to acknowledge that our participants provided blood samples at various testing locations and different times following the eye-tracking protocol. Because we were unable to collect serum hormone concentrations at the time of testing, we are unable to draw conclusions about any immediate causal effects of hormone variation on visual fixations at the time of testing. Relatedly, it is possible that variability within participants’ testing procedures and/or experiences between testing and serum collection could have influenced our results. This limitation underlies our choice to primarily analyze total rather than free (i.e. unbound) T levels, because concentrations of the latter change rapidly and often. We also did not assess other androgens that may influence sexual motivation in men, such as dihydrotestosterone (DHT). Although data suggesting a link have been inconsistent [e.g. Citation44], including DHT in future studies would help to clarify any potential role.
Conclusion
Taken together, we show here that both SHBG and E2 may play roles in male visual attention, which could impact sexual cognition. In addition to having broad implications for male sexual behavior in general, our findings are also relevant to quality of life of PCa patients, who are on ADT and are deprived of not only T, but also of E2. As part of informed consent for PCa treatment through ADT, PCa patients could benefit from being told how the treatment might influence their visual attention and that this could be a result of both loss of T and E2. Further, increasing SHBG levels with age may have cognitive impacts that affect various activities of daily living, including sexual function. These hypotheses warrant further investigation.
Author contributions
JPH designed the study, supervised data collection, ran data analysis, interepreted results, and wrote the manuscript. STSW recruited participants, collected data, managed data entry, and reviewed the manuscript. RJW designed the study, recruited participants, supervised data collection, interepreted results, and also contributed to wroting the manuscript. AK provided the eye-tracking protocol/stimuli, equipment, and technical assistance, assisted with study design and interpretation, and reviewed the manuscript. EW contributed to designing the study, assisted with recruiting participants and collecting data, assisted with data analysis, interpreted the results, and wrote the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Excel (17.7 KB)Acknowledgments
We thank the prostate cancer support groups and community centers in British Columbia who helped us in recruiting participants. We also thank L. Forby, S. Milani, C. Krebs, MD/PhD, and B. Lee for their technical and procedural assistance during this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Study data are available in Supplementary Materials.
Additional information
Funding
References
- Janssen E. Sexual arousal in men: a review and conceptual analysis. Horm Behav. 2011;59(5):708–716.
- Janssen E, Everaerd W, Spiering M, et al. Automatic processes and the appraisal of sexual stimuli: toward an information processing model of sexual arousal. J Sex Res. 2000;37(1):8–23.
- Kacker R, Traish AM, Morgentaler A. Estrogens in men: Clinical implications for sexual function and the treatment of testosterone deficiency. J Sex Med. 2012;9(6):1681–1696.
- Bagatell CJ, Heiman JR, Rivier JE, et al. Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J Clin Endocrinol Metab. 1994;78(3):711–716.
- Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: Clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185(1):17–23.
- Finkelstein JS, Lee H, Burnett-Bowie SAM, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022.
- Watts TM, Holmes L, Savin-Williams RC, et al. Pupil dilation to explicit and non-explicit sexual stimuli. Arch Sex Behav. 2017;46(1):155–165.
- Wenzlaff F, Briken P, Dekker A. Video-based eye tracking in sex research: a systematic literature review. J Sex Res. 2016;53(8):1008–1019.
- Lykins AD, Meana M, Strauss GP. Sex differences in visual attention to erotic and non-erotic stimuli. Arch Sex Behav. 2008;37(2):219–228.
- Nummenmaa L, Hietanen JK, Santtila P, et al. Gender and visibility of sexual cues influence eye movements while viewing faces and bodies. Arch Sex Behav. 2012;41(6):1439–1451.
- Dixson BJ, Grimshaw GM, Linklater WL, et al. Eye-tracking of men's preferences for waist-to-hip ratio and breast size of women. Arch Sex Behav. 2011;40(1):43–50.
- Garza R, Heredia RR, Cieslicka AB. Male and female perception of physical attractiveness: an eye movement study. Evol Psychol. 2016;14(1):147470491663161.
- Rieger G, Cash BM, Merrill SM, et al. Sexual arousal: the correspondence of eyes and genitals. Biol Psychol. 2015;104:56–64.
- Huberman JS, Maracle AC, Chivers ML. Gender-specificity of women's and men's self-reported attention to sexual stimuli. J Sex Res. 2015;52(9):983–995.
- Rupp HA, Wallen K. Sex differences in viewing sexual stimuli: an eye-tracking study in men and women. Horm Behav. 2007;51(4):524–533.
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92(10):3844–3853.
- Kampen DL, Sherwin BB. Estradiol is related to visual memory in healthy young men. Behav Neurosci. 1996;110(3):613–617.
- Salminen EK, Portin RI, Koskinen AI, et al. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer. 2005;103(7):1381–1387.
- Pérez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145(1):117–139.
- Simerly RB, Swanson LW, Chang C, et al. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study . J Comp Neurol. 1990;294(1):76–95.
- Krauzlis RJ, Lovejoy LP, Zénon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182.
- Elliott S, Latini DM, Walker LM, ADT Survivorship Working Group, et al. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996–3010.
- Wibowo E, Wassersug RJ, Robinson JW, et al. How are patients with prostate cancer managing androgen deprivation therapy side effects? Clin Genitourin Cancer. 2019;17(3):e408–e419.
- Caldwell J, Jirikowski G. Sex hormone binding globulin and aging. Horm Metab Res. 2009;41(3):173–182.
- Keevil BG, Adaway J. Assessment of free testosterone concentration. J Steroid Biochem Mol Biol. 2019;190:207–211.
- Palmer-Hague JL, Tsang V, Skead C, et al. Androgen deprivation alters attention to sexually provocative visual stimuli in elderly men. Sex Med. 2017;5(4):e245–e254.
- Nasiopoulos E, Risko EF, Foulsham T, et al. Wearable computing: will it make people prosocial? Br J Psychol. 2015;106(2):209–216.
- Risko EF, Kingstone A. Eyes wide shut: implied social presence, eye tracking and attention. Atten Percept Psychophys. 2011;73(2):291–296.
- McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona sexual experience scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40.
- Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905.
- Radloff LS, The CES. D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401.
- McCreary DR, Saucier DM, Courtenay WH. The drive for muscularity and masculinity: testing the associations among gender-role traits, behaviors, attitudes, and conflict. Psychol Men Masculinity. 2005;6(2):83–94.
- Ishihara S. Tests for color blindness. Am J Ophthalmol. 2918;1:376.
- Wibowo E, Wassersug RJ. Does the timing of estrogen administration after castration affect its ability to preserve sexual interest in male rats? Exploring the critical period hypothesis. Physiol Behav. 2013;110–111:63–72.
- Kruijver FPM, Balesar R, Espila AM, et al. Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol. 2003;466(2):251–277.
- Alexander GM, Sherwin BB. The association between testosterone, sexual arousal, and selective attention for erotic stimuli in men. Horm Behav. 1991;25(3):367–381.
- Brooks DC, Zhao H, Coon VJ, et al. Brain aromatase is essential for regulation of sexual activity in male mice. J Endocr Soc. 2020;4:1–15.
- Kisch H, Gross S, Wallaschofski H, et al. Associations of androgens with depressive symptoms and cognitive status in the general population. PLOS One. 2017;12(5):e0177272.
- Muller M, Schupf N, Manly JL, et al. Sex hormone binding globulin and incident Alzheimer's disease in elderly men and women. Neurobiol Aging. 2010;31(10):1758–1765.
- Hsu B, Cumming RG, Waite LM, et al. Longitudinal relationships between reproductive hormones and cognitive decline in older men: the concord health and ageing men project. J Clin Endocrinol Metab. 2015;100(6):2223–2230.
- Lin KA, Rundel C, Doraiswamy MP, for the Alzheimer’s Disease Neuroimaging Initiative. Serum SHGB levels are not associated with longitudinal cognitive decline in mild cognitive impairment. JAD. 2016;55(3):1123–1130.
- Tran S, Walker LM, Wassersug RJ, et al. What do canadian uro-oncologists believe patients should know about androgen deprivation therapy? J Oncol Pharm Pract. 2014;20(3):199–209.
- Wibowo E, Wassersug RJ, Robinson JW, et al. An educational program to help patients manage androgen deprivation therapy side effects: feasibility, acceptability, and preliminary outcomes. Am J Mens Health. 2020;14(1):1557988319898991.
- Corona G, Isidori AM, Aversa A, et al. Endocrinologic control of men's sexual desire and arousal/erection. J Sex Med. 2016;13(3):317–337.