Abstract
Introduction
The androgen receptor (AR) mediates peripheral effects of testosterone. Evidence suggests that the number of CAG repeats in exon-1 of the AR gene negatively correlates with AR transcriptional activity. The aim of this analysis was to determine the association between CAG repeat number and mortality in men.
Methods
Men aged 40–79 years were recruited from primary care for participation in the UK arm of the European Male Aging Study between 2003 and 2005. Cox proportional hazards modelling was used to determine the association between CAG repeat number/mortality. Results were expressed as hazard ratios(HR)/95% confidence intervals (CI).
Results
312 men were followed up. The mean baseline age was 59.5 years. At follow up, 85/312(27%) men had died. CAG repeat length ranged from 14 to 39, with the highest proportion of CAG repeat number at 21 repeats(16.4%). In a multivariable model, using men with CAG repeat numbers of 22-23 as the reference, men with a lower number of CAG repeats(<22) showed a trend for a higher mortality in the follow-up period (HR 1.46 (0.75, 2.81)) as did men with higher number of repeats (>23) (1.37 (0.65, 2.91)).
Conclusion
Our data suggest that CAG repeat number may partially influence the risk of mortality in men. Further larger studies are required to quantify the effect.
Keywords:
Introduction
The peripheral effects of testosterone are mediated through the androgen receptor (AR). A number of studies have shown an association between a low serum testosterone (T) and increased all-cause and cardiovascular-related mortality, although a previous meta-analysis of 12 community-based surveys revealed considerable inconsistency between individual studies [Citation1]. Whether low T level is a non-specific risk marker of poor health [Citation1,Citation2] or the association is mediated by the effects of T deficiency on the cardiovascular system [Citation3] is currently unclear. Erectile dysfunction (ED) is increasingly recognized as an early warning signal of impending cardiovascular disease and a predictor of excessive mortality [Citation3–5].
Late onset hypogonadism (LOH) is defined as the simultaneous occurrence of three sexual symptoms (decreased sexual interest and morning erections plus erectile dysfunction) and circulating total Testosterone (T) level below 11 nmol/L plus free T below 220 pmol/L [Citation6]. Using these criteria, associations have been reported between LOH and a variety of end organ deficits suggestive of androgen deficiency [Citation7].
Pye et al. [Citation8], in an earlier analysis of the European Male Ageing Study (EMAS) cohort, using a combination of deaths verified from death certificates (25%), death registers (37%), medical/hospital records (27%) or from the reporting of a family member or contact person, reported that severe LOH was associated with substantially higher risks of all-cause and cardiovascular mortality, to which both the level of T and the presence of sexual symptoms contribute independently.
The androgen receptor (AR) mediates the peripheral effects of testosterone. The main mechanism of action for the AR is to direct regulation of gene transcription. Exon 1 of the AR gene contains a polymorphic sequence of CAG repeats, which varies in number from 10 to 35, and which encodes polyglutamine stretches of the AR transactivation domain [Citation9]. The evidence suggests that the number of CAG repeats is negatively correlated with the transcriptional activity of the AR [Citation9–11].
We have previously shown in a male cohort of people with type 2 diabetes (T2DM) that a “u” shaped curve relation existed between number of CAG repeats and mortality [Citation12]. The hypothesis developed was that there was an optimal CAG repeat number in relation to sensitivity of the androgen receptor, which relates to the effects of testosterone on metabolically active tissues and on the vascular system and that men with a CAG repeat number above or below this, may experience a less favorable long-term cardiovascular outcome. In an earlier meta-analysis, it was reported that men with <22 and >23 CAG repeats had an approximately 20% higher risk of being infertile than men with 22 or 23 CAG repeats [Citation13].
In the light of these findings, our aim was to determine if any relation existed between the number of CAG repeats and all-cause mortality in a United Kingdom (UK) prospective cohort of mostly Caucasian men recruited from primary care [Citation14], taking into account baseline testosterone level.
Materials and methods
Participants were recruited from a primary care register within Greater Manchester for participation in the European Male Aging Study between 2003 and 2005 [Citation8]. The men participating completed a baseline questionnaire and had a fasting blood sample. Subsequent deaths were assessed by review of the participants’ medical care record up to the end February 2021 [Citation15]. Ethical permission for the study was granted by the North West of England Multi-centre Research Ethics Committee, REC registration number: 01/8/095.
Postcode derived from the address of the participant in the general practice records was used to derive the Index of Multiple Deprivation (IMD) [Citation16].
Hormones and biochemistry
A single fasting morning (venous blood sample (before 10 am) was obtained from each participant. The blood samples were collected between 2003 and 2005.
The testosterone level was measured by liquid chromatography–mass spectrometry (LC-MS) as described previously [Citation17]. Sex Hormone Binding Globulin (SHBG) was measured by the Modular E170 platform electrochemiluminescence immunoassay (Roche Diagnostics). Free T levels were derived from total T, SHBG, and albumin concentrations by the Vermeulen formula [Citation18]. Measurement of total estradiol was carried out by gas chromatography-tandem mass spectrometry [Citation17].
Determination of CAG repeat number
Genetic analysis was done in 2008. DNA extracted from whole blood was subjected to polymerase chain reaction (PCR) to amplify the region of the AR gene containing AR exon 1 CAG triplet repeat. PCR preparation, primers, and conditions were as described in a previous study [Citation7]. Genotyping of the CAG repeat was carried out in the laboratory of the Centre for Integrated Genomic Medical Research (The University of Manchester), using fluorescently-labeled PCR. Ten nanograms of DNA were amplified in 10-l reactions containing 2.5 pmol each of fluorescently labeled forward and reverse primer, 10 PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, and 0.2 U Taq DNA polymerase. The primer sequences were: forward, 5-TCC AGA ATC TGT TCC AGA GCG TGC-3; and reverse, 5-GCT GTG AAG GTT GCT GTT CCT CAT-3. Reactions were cycled at 95 °C for 5 min; 10 cycles of 94 °C for 10 s, 55 °C for 30 s, and 72 °C for 30 s; 20 cycles of 89 °C for 20 s, 55 °C for 30 s, and 72 °C for 30 s; and finally, 72 °C for 10 min. Samples were then run on an ABIPRISM 3100 Genetic Analyser (Applied Biosystems, Foster City, CA) and genotyped using Genescan (Applied Biosystems). Allele frequencies were checked for consistency with HapMap data or literature where possible.
Statistical analysis
Cox regression was used to determine the association between number of CAG repeats and all-cause mortality. Participants contribute person-time from the date of recruitment to death and stopped contributing at their date of death, or end of February 2021. Based on previously published thresholds [Citation13], CAG repeats were categorized as <22, 22-23 (referent group) and >23. Analyses were adjusted for age, decile of Index of Multiple Deprivation, total testosterone at baseline, and estradiol. In a separate model, we also adjusted additionally for SHBG.
To determine whether the association between AR CAG repeats and mortality varied by level of total testosterone, we categorized total testosterone into tertiles and repeated the Cox regression analysis for participants in each of the three tertiles of total testosterone, in separate models. In the models stratified by tertile of total testosterone, we adjusted for total testosterone as a continuous variable.
Results
In total, 396 men were recruited to the study of whom 312 had complete data for both CAG repeat and mortality. At baseline, 22/312 reported having diabetes (7.16%). The age range at recruitment of the 312 men who contributed data for the analysis was 40–80 years (mean 59.5 years). 93% of participants in the study were of Caucasian ethnicity. During a follow up period, of up to 17 years 85/312 (27.2%) of men had died. Baseline data are summarized in .
Table 1. Participant characteristics.
CAG repeat distribution
In this group, the highest proportion of CAG repeats occurred for the 19 repeating motif (44/312 men, 14.1%), 20 repeats (48/312 men, 15.4%)), and 21 repeats (51/312 men, 16.4%). The number of CAG repeats varied between 14 and 39 ().
Table 2. Distribution of CAG repeats.
Relation between CAG repeat number and mortality
Compared to participants with 22-23 CAG repeats, participants with <22 and >23 CAG repeats, respectively, had a higher hazard ratio (95% CI) for all-cause mortality during the study period in a model adjusted for age, total testosterone, total estradiol, and decile of index of multiple deprivation, though this was not statistically significant at the 95% confidence level, 1.46 (0.75, 2.81), 1.37 (0.65, 2.91) ( and ). SHBG attenuated the association between number of CAG repeats and mortality ().
Figure 1. Kaplan–Meier plot showing cumulative mortality in each CAG group.
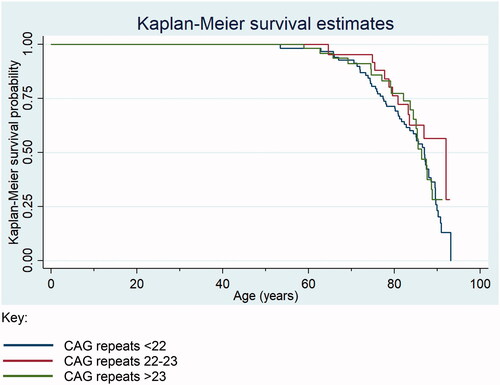
Table 3. Association between number of CAG repeats and all-cause mortality.
The association between AR CAG repeats and mortality varied by tertile of total testosterone at baseline. In a model adjusted for age, total testosterone (continuous measure), total estradiol, and quintile of index of multiple deprivation including only men in the lowest tertile of total testosterone at baseline, the hazard ratio (95% CI) for mortality among those with <22 and >23 CAG repeats, respectively (compared to 22-23 CAG repeats was) 2.64 (0.76, 9.13) and 2.65 (0.66, 10.66). The corresponding results among men in the middle tertile of total testosterone was 1.72 (0.35, 8.45) and 1.76 (0.34, 9.13) and among men in the highest tertile of testosterone was 1.03 (0.34, 3.12) and 0.87 (0.24, 3.13).
Discussion
In this long-term prospective follow-up study (approximately 17 years), we observed a trend for men with a CAG repeat number less than 22, or more than 23 for increased likelihood to die over the period of follow-up than those with a CAG repeat number between 22 and 23. This effect was modified by serum testosterone level at baseline with a diminishing effect by increasing tertile of serum testosterone level (baseline). This is the longest follow-up study to describe this phenomenon and accords with the findings of a recently published study in T2DM men where a similar but not identical association was seen [Citation12]. Interestingly among men with type 2 diabetes, in the study of Wong et al., a higher total testosterone was reportedly associated with increased mortality in the presence of shorter CAG repeat length but decreased mortality in those with long CAG repeats [Citation19].
A smaller effect size in the relation between CAG repeat number and mortality was seen here than was previously described in T2DM men [Citation12]. The reduced effect size may relate to an increased cardiovascular event rate in men with T2DM than the male participants in this study and the fact that the men with T2DM were older at recruitment than the men whose outcomes were reported here. Nevertheless the direction was similar.
An increasing CAG repeat number within exon 1 of the AR gene polymorphism has previously been linked to increased AR insensitivity [Citation9–11]. In a cohort of T2DM men, we previously reported a “u” shaped relation between the number of CAG repeats and mortality, such that presence of 21 CAG repeats was associated with up to an 58% lower mortality rate than <20 CAG repeats and >21 CAG repeats [Citation12]. While the findings of this current study slightly differ, they are in an independent sample of men of whom 6.8% reported having diabetes at baseline. The fact that the relation between CAG repeat length and mortality in a general population sample of men was less strong than in men with type 2 diabetes does raise the question of whether the effect may be greater in men with an underlying predisposition to cardiovascular disease. The numbers here were not sufficient to look at this as only 6.8% of the men had a diagnosis of T2DM at recruitment.
The transactivational activity of the AR is known to be inversely associated with the number of CAG repeats [Citation20]. Whether variations of the CAG repeat lengths within a particular range are associated with clinical changes in tissue androgenization is uncertain. Similarly, there is no conclusive evidence that AR CAG repeat polymorphisms modulate the responsiveness to exogenous testosterone replacement in hypogonadism. Larger studies are required to characterize fully the impact of modest variability in CAG repeat length on mortality in men, as well as to more precisely to define the interaction between CAG repeat number and circulating testosterone/SHBG concentration in modulating mortality risk in men.
Independent associations between AR exon 1 CAG length and adverse cardiovascular risk factors, such as high LDL-cholesterol [Citation21], low HDL-cholesterol [Citation22], and high blood pressure [Citation8,Citation22], have been demonstrated by other studies. Specifically association between AR exon 1 longer CAG repeat length and low total testosterone concentrations appears to exert an adjunctive worsening effect on the metabolic profile [Citation23,Citation24]. This suggests some level of complexity of the role of the CAG polymorphism in regulating the relation between androgen effects and cardiovascular risk factors, which may inform future risk calculators taking into account CAG repeat number. At this time, there are no reports to suggest that CAG repeat number is itself associated with the likelihood of a man developing T2DM.
It is relevant to risk factors for early death reported that men with CAG repeat length below 21 or above 24 had a, respectively, 50% and 76% higher risk of testicular cancer than patients with CAG repeat number of 21–24 [Citation25]. In other words, the risk of developing testicular cancer would seem to be lower for men with a CAG repeat number between 21 and 24. In another study with an approach similar to the study reported here (as mentioned in the Introduction), a meta-analysis of 3915 men (1831 fertile and 2084 infertile) [Citation13] reported that men with <22 and >23 CAG repeats had an approximately 20% higher risk of being infertile than men with 22 or 23 CAG repeats [Citation13]. Taken together with our findings, the evidence supports the notion that normal AR function is sustained over a critical but limited range of CAG repeat number.
At this point in time, we do not have access to the actual cause of death [Citation26]. Cause specific mortality was not available to us. Further work is needed to at look potential associations between CAG repeat number and specifically cardiovascular mortality. This is a relatively small sample and any specific recommendations/guidance would require a larger analysis. We anticipate that with corroboration from other cohorts it may be possible more precisely to define the interaction between CAG repeat number and circulating testosterone/SHBG concentration in modulating mortality risk in men.
Strengths/limitations
A strength of the paper is the duration of follow-up of the participants. A limitation of the paper is that we have only analysed the UK subset of the EMAS cohort, since linked NHS data were available for this subset. Therefore out analysis is limited by sample size. A further limitation is that we only have mortality data, not any details of comorbidities as at 2019/2020 that may have developed over time. We plan to obtain this information in the future and also to extend the cohort analysis to the other cohorts of the EMAS study.
We accept that there are many other risk factors for cardiovascular disease. However these have been linked to relative “functional” hypogonadism which is the consequence of androgen receptor resistance secondary to a low or high number of CAG repeats. With a relatively small number of deaths, we did not feel that inclusion of multiple linked cardiovascular risk factors would be appropriate here.
Regarding the analysis of the association between prescribing over time/development of neoplastic disorders and mortality, this would require a larger cohort and a complex longitudinal analysis which we are in the process of putting together. Finally we accept that the study was conducted mainly in Caucasian men.
This is a relatively small sample and any recommendations regarding CAG repeat measurement would require a larger analysis. We anticipate that with corroboration from other cohorts it may be possible more precisely to define the interaction between CAG repeat number and circulating testosterone/SHBG concentration in modulating mortality risk in men.
Conclusions
In this long-term prospective follow-up study, we observed that men with an AR exon CAG repeat number less than 22 or more than 23 showed a trend towards a higher mortality rate over the period of follow-up than those with a CAG repeat number between 22 and 23. These results did not reach levels of formal statistical significance. The effect was greater in men with a lower baseline testosterone level.
A greater understanding of the interaction between CAG repeat number and circulating testosterone level could add further to our understanding of the endocrine processes that modulate mortality risk in men.
Disclosure statement
None of the authors has any conflict of interest regarding this study.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
Notes on contributors
Adrian Heald
Adrian Heald is a consultant physician in the NHS and senior research fellow at Manchester University.
Michael Cook
Michael Cook is a final year PhD student at Manchester University.
Leen Antonio
Leen Antonio is a clinical endocrinologist and researcher based in Leuven.
Dirk Vanderschueren
Dirk Vanderschueren is a professor of medicine and research group leader in the Laboratory of Clinical and Experimental Endocrinology at Leuven.
Ahmed Javed
Ahmed Javed is a clinical fellow at Northampton General Hospital in the UK.
Helene Fachim
Helene Fachim is a post-doctoral researcher at Manchester University.
Geoff Hackett
Geoff Hackett is a professor of Men's Health at the University of Aston, Birmingham.
Fred Wu
Fred Wu is a professor of Endocrinology at Manchester University and Terence O’Neill is Consultant Rheumatologist in the NHS and Professor of Rheumatology and Clinical Epidemiology at Manchester University.
References
- Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):167–3019.
- Ruige JB, Mahmoud AM, De Bacquer D, et al. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870–875.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701.
- Araujo AB, Travison TG, Ganz P, et al. Erectile dysfunction and mortality. J Sex Med. 2009;6(9):2445–2454.
- Corona G, Monami M, Boddi V, et al. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010;7(4):1557–1564.
- Wu FC, Tajar A, Beynon JM, EMAS Group, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135.
- Tajar A, Huhtaniemi IT, O'Neill TW, EMAS Group, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508–1516.
- Pye SR, Huhtaniemi IT, Finn JD, EMAS Study Group, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99(4):1357–1366.
- Stanworth RD, Kapoor D, Channer KS, et al. Androgen receptor CAG repeat polymorphism is associated with serum testosterone levels, obesity and serum leptin in men with type 2 diabetes. Eur J Endocrinol. 2008;159(6):739–746.
- Tirabassi G, Cignarelli A, Perrini S, et al. Influence of CAG repeat polymorphism on the targets of testosterone action. Int J Endocrinol. 2015; 2015:298107.
- Stanworth RD, Kapoor D, Channer KS, et al. Dyslipidaemia is associated with testosterone, oestradiol and androgen receptor CAG repeat polymorphism in men with type 2 diabetes in men with type 2 diabetes. Clin Endocrinol (Oxf). 2011;74(5):624–630.
- Heald AH, Yadegar Far G, Livingston M, et al. Androgen receptor-reduced sensitivity is associated with increased mortality and poorer glycaemia in men with type 2 diabetes mellitus: a prospective cohort study. Cardiovasc Endocrinol Metab. 2020;10(1):37–44.
- Nenonen HA, Giwercman A, Hallengren E, et al. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility – a meta-analysis. Int J Androl. 2011;34(4pt1):327–332.
- Lee DM, O'Neill TW, Pye SR, EMAS Study Group, et al. The European Male Ageing Study (EMAS): design, methods and recruitment. Int J Androl. 2009;32(1):11–24.
- Available from: https://digital.nhs.uk/services/summary-care-records-scr. Accessed 14 February 2021.
- Available from: https://imd-by-postcode.opendatacommunities.org/imd/2019. Accessed 15 February 2021.
- Labrie F, Bélanger A, Bélanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4–5):182–188.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Wong PW, Lee HM, Lau ESH, et al. Interactive effects of testosterone and the androgen receptor CAG repeat length polymorphism on cardiovascular-renal events and mortality in men with diabetes. Diabetes Metab Res Rev. 2019;35(1):e3081.
- Choong CS, Wilson EM. Trinucleotide repeats in the human androgen receptor: a molecular basis for disease. J Mol Endocrinol. 1998;21(3):235–257.
- Trzmiel-Bira A, Filus A, Kuliczkowska-Płaksej J, et al. Polimorfizm CAG genu receptora androgenowego a wystepowanie wybranych parametrów zespołu metabolicznego u mezczyzn w wieku 45-65 lat w populacji wrocławskiej [The CAG repeat polymorphism in androgen receptor gene repeat and frequency of chosen parameters of metabolic syndrome in 45–65 aged men in Wroclaw population]. Endokrynol Pol. 2008;59:477–482.
- Zitzmann M, Gromoll J, von Eckardstein A, et al. The CAG repeat polymorphism in the androgen receptor gene modulates body fat mass and serum concentrations of leptin and insulin in men. Diabetologia. 2003;46(1):31–39.
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92(10):3844–3853.
- Haring R, Ernst F, Schurmann C, et al. The androgen receptor CAG repeat polymorphism as a risk factor of low serum testosterone and its cardiometabolic effects in men. Int J Androl. 2012;35(4):511–520.
- Grassetti D, Giannandrea F, Paoli D, et al. Androgen receptor polymorphisms and testicular cancer risk. Andrology. 2015;3(1):27–33.
- Available from: https://digital.nhs.uk/services/spine. Accessed 8 February 2022.
