Abstract
Context
Testosterone (T) therapy of hypogonadal men requires stable kinetics, tolerance and attenuation of symptoms. Both intramuscular injections of the long-acting ester T undecanoate (TU) and transdermal application of T gel offer a proven efficacy. As T has marked effects on hematopoiesis, an elevation of hematocrit has to be considered during T therapy.
Objective
To compare the effects of a transdermal T gel with long-acting intramuscular TU on hematopoiesis, controlling for age, diagnosis, androgen receptor susceptibility and obesity.
Design
Prospective two-arm open registry, minimum duration of 26 weeks per patient. Putative modulators of erythropoiesis entering regression models were type of medication, type of hypogonadism, delta of total testosterone concentrations, waist circumference, age as well as (in a sub-group) androgen receptor gene CAG repeat length.
Setting
Tertiary university based andrological outpatient department.
Patients
802 hypogonadal men, 498 receiving T gel and 304 receiving intramuscular TU, median age 40 years (interquartile range = 25).
Results
Follow-up visits after initiation of treatment occurred between treatment weeks 26-30. Serum T concentrations increased markedly in both patient groups. Men receiving intramuscular TU exhibited an increased hematocrit (>50%) to a significantly higher amount than men receiving T gel (69/304 vs. 25/498, p < 0.001). Corresponding results were seen for higher values of hematocrit (>52% and >54%). Advanced age (p = 0.009), higher waist circumference (p = 0.01), higher delta testosterone (p = 0.007) and functional vs classical hypogonadism (p = 0.04) contributed to the effect in stepwise multiple regression models. Attenuated androgen action (longer androgen receptor CAG repeats) mitigated the effect (p = 0.01) in a subgroup of 574 patients. Men with anemia (hemoglobin ≤12.7 g/dl) were more likely to move out of the pathological range when receiving TU vs T gel (41/53 vs. 49/89 p = 0.01).
Conclusions
T substitution with intramuscular TU or T gel increase T concentrations effectively. Long-acting TU leads to a higher rate of hematocrit levels >50%, whilst at the same time it seems to be more efficient to ameliorate anemia in the subgroup of respectively affected hypogonadal patients. This applies especially to obese older men with functional hypogonadism.
Introduction
Hypogonadism affects about 5% of the male population, characterized by low serum testosterone (T) levels in association with symptoms including fatigue, decreased libido, erectile dysfunction, depression, decreased muscle mass and bone density as well as anemia [Citation1–4]. While generally ameliorating these symptoms of hypogonadism and being able to revert a state of anemia [Citation1–3], T replacement therapy (TRT) can lead to side effects of potentially adverse nature, with increased hematocrit being the paramount and most often described one [Citation1–16]. Increased hematocrit, in turn, may promote cardiovascular events (stroke, myocardial ischemia) [Citation1–16].
To date, it is unclear whether various forms of TRT, such as transdermal gels or injectable esters of T contribute to a different extent to the development of elevated hematocrit. There are indications from a retrospective data analysis performed in the US [Citation14] that injectable forms of TRT lead to higher serum concentrations of testosterone and also to a higher rate of increased hematocrit (defined as >50%), namely in 65% of patients vs. 13% of patients treated with transdermal gels. However, these retrospective data refer to short-acting T esters. Rather, regarding invasive forms of TRT, long-acting injectable T undecanoate has become a very common formulation. A recent trial involving almost 1000 men receiving long-acting injections of intramuscular T undecanoate vs placebo reported a high rate of increased hematocrit, a safety trigger for hematocrit greater than ≥54% occurred in six (1%) of 484 participants in the placebo group and 106 (22%) of 491 participants in the testosterone group [Citation15]. This form of treatment of was not directly compared to another transdermal form and the study population was restricted to elderly obese men, exhibiting metabolic risk factors [Citation15].
Generally, most common forms of TRT, at least in Europe, are transdermal T preparations (T gels) which can be applied, e.g. in daily doses of one to four times of 20.25 mg to the skin and the other widespread form of treatment is using injectable T undecanoate 1000 mg (intramuscular application every 10–14 weeks). Titration of T levels by using transdermal gels is performed by modulating the dose, while using the injectable forms, the injection intervals are changed according to the patient’s T levels.
To date, it remains unclear, whether effects on erythropoiesis and hematocrit are different between those two types of treatment, while it seems to be clear that short-acting esters of testosterone bear an increased risk for critical elevation of hematocrit compared to transdermal gels [Citation14,Citation16]. It is also unclear to what extent age, body mass index, initial values of serum testosterone concentration and the change in serum testosterone concentrations influence the hematopoietic effect of T treatment. The androgen receptor gene CAG repeat polymorphism (longer repeats attenuate T function and have been reported to be associated with mitigated effects of androgens in hematopoiesis) might further modulate the effects [Citation13]. The mechanisms by which T exerts effects on hematopoiesis are not fully understood. This could be exerted via erythropoietin secretion, direct effects on bone marrow progenitor cells and effects on iron sequestration and turnover. In addition, hepcidin metabolism is debated to play a pivotal role [Citation13,Citation16–19].
Registries that enroll well-characterized patients, such as TRiUS [Citation20] or RHYME [Citation21] in the case of male hypogonadism, may provide observational data with prospectively defined high quality for guiding clinical decision-making.
Aims and hypotheses of this prospective registry
Primary aim
To assess the effects of TRT by either transdermal topical T gel vs. injectable long-acting T undecanoate on hematocrit and hemoglobin levels. In detail, amelioration of anemia (defined as increase of hemoglobin of ≥1 g/dl if baseline ≤12.7 g/dl [Citation3], as well as eliciting safety triggers of erythrocytosis, defined as hematocrit increases >50%, >52%, >54% and > 56%.
Secondary aim
To determine factors that influence the hematopoietic response to testosterone in hypogonadal men (age, BMI or waist circumference, baseline testosterone levels, AR gene CAG repeats, type of hypogonadism [primary or secondary vs functional hypogonadism]).
Hypothesis
Based on previous retrospective data, it is assumed that the transdermal form of TRT leads to a lower extent of elevated hematocrit vs the injectable form of treatment.
Site and patients
Prospective mono-centric registry for TRT including hypogonadal men for a minimum treatment period of 26 weeks per person.
Treatment site: Centre for Reproductive Medicine and Andrology/Clinical and Surgical Andrology, University Clinics Muenster, Germany. All subjects provided written informed consent to the analysis of serum as approved by the Ethics Committee of the University and the State Medical Board (codes 2009-164-S; 2013-255-f-S).
Inclusion criteria
Post-pubertal men (aged 25–65 years) in general good health with symptoms of hypogonadism and total serum testosterone below 12.1 nmol/L or free serum testosterone below 243 pmol/L according to EAU guidelines [Citation22]. Informed consent to initiate testosterone therapy and to be included in a patient registry for a treatment duration of at least one year using either T gel by pump device (20.25 mg of T per hub) or T undecanoate 1000 mg by intramuscular injection. As this is an observational registry, the formulation and the dosing scheme are determined independently by the prescribing physician in agreement with the patient. Control (follow-up) visits had to take place within the time interval of 26 to 30 weeks after initiation of treatment according to standard care to qualify a patient to be part of the registry. Additional visits were possible.
Diagnosis of type of hypogonadism (primary, secondary or functional) was performed according to EAU [Citation22] and EAA guidelines [Citation1].
Exclusion criteria
History of prostate cancer, PSA >4 ng/mL, conspicuous findings upon digital rectal exam (DRE), feminizing testicular or adrenal tumors, men with an unstable, uncontrolled or severe medical disease (i.e. CHF NYHA >2, COPD > Gold 3), liver cirrhosis, any end-stage kidney disease, uncontrolled hypo- or hyperthyroidism, chronic infections, polycythemia, substance abuse including doping with androgens, or other contraindication to TRT, such as, but not limited to, present or actual wish or paternity.
Men with suspicion of mammary cancer by ultrasound, history or clinical impression. Elevated levels of hemoglobin (>18 g/dL) or hematocrit (>50%) before treatment. Poorly controlled diabetes mellitus of type 1 or Type 2 (HbA1c >9%). Transsexual men/men with gender dysphoria with or without any kind of treatment.
Current treatment or treatment stopped within 3 months before putative inclusion into the trial with finasteride, spironolactone, hCG, ketoconazole, cimetidine, raloxifene, antiestrogens/SERMS, aromatase inhibitors, clomiphene citrate, testosterone preparations, dihydrotestosterone, estrogens, progesterone, synthetic progestins, LHRH agonists, GnRH antagonists.
Treatment allocation and treatment
Patient care and patient allocation was according to a registry of daily clinical care practice. That means that patients did not follow a rigid study regimen but a normal standard of care, which requires them to see the treating physician within a time-period of about 6 months after initiation of treatment. Patients were allocated as detailed above as they appeared and started treatment. A time of 3 years was estimated as adequate to find and allocate the required number of patients (see power calculation) to the registry and keep them within the registry for at least 26 weeks per person. This time setting was chosen because changes in erythropoiesis seem to be stable after such a time period of T treatment according to previous data [Citation14]. TRT with T gel or with injectable T undecanoate were the choice of the patient after being counseled by the prescribing physician.
Adaptation of treatment doses was not performed during data acquisition within the relevant time period of 26-30 weeks. T gel was applied daily with 2 hubs of 20.25 mg T each from a dosing device. To achieve a steady-state in patients using intramuscular injections of testosterone undecanoate, following initiation of therapy, the second injection was given after 6 weeks. Thereafter, the dosing interval was prolonged to 12 weeks. In general, patients were injected according to the following regimen: week 0→week 6→week 18→week 30. These patients were seen shortly before the 4th injection for control.
Change of TRT from gel to injection or vice versa led to exclusion of subjects from analysis. Data were recorded by treating physicians and study nurses in the local secure database. Serum was analyzed on site and stored for later analysis.
Laboratory assessments
All venous blood samples were obtained between 08:00 and 12:00 am in the morning. Patients using transdermal gel had to apply T gel 2–4 h before sampling. Serum or plasma were separated at 800 g. Samples were analyzed after collection or snap frozen and stored at −20 °C. Serum samples were obtained 4–6 h after application of T gel or as nadir levels shortly before the next injection of T undecanoate.
Serum testosterone levels were measured by a commercial ELISA kit (DRG Instruments GmbH, Marburg, Germany). This immunoassay for testosterone is calibrated quarterly against standards using liquid chromatography – mass spectroscopy (LCMS-MS), the immunoassay regularly passes this quality check and reproduces the results of mass spectroscopy with an imprecision of <10% in the range for serum testosterone concentrations between 5 and 20 nmol/L. Intra-assay coefficients of variation (CV) were below 2%, mean inter-assay CVs below 5%.
Concentrations of sex hormone binding globulin (SHBG) were determined by highly specific time-resolved fluoroimmunoassays (Autodelfia, Freiburg, Germany). Mean intraassay coefficients of variation (CV) were below 5%, mean interassay CVs below 10%. Levels of free testosterone were calculated from levels of SHBG and total serum testosterone according to previously published calculations [Citation23].
Serum concentrations of FSH and LH were determined using highly specific time-resolved fluoro-immunoassays (Autodelfia, Freiburg, Germany). Mean intra-assay CV were below 2% and mean inter-assay CVs below 5%. Proteo-hormone assays are under quarterly blinded external quality control, as well and pass regularly.
Red blood count was performed on a Sysmex SE 9500 system (Sysmex Europe, Hamburg, Germany).
DNA was isolated from EDTA blood samples using the Nucleon Kit (Amersham Life Science, Freiburg, Germany) and analysis of the AR gene microsatellite residues was performed as previously published [Citation24]. This was done in a subset of patients who agreed to genetic analyses (n = 574).
Statistics
Sample size calculation
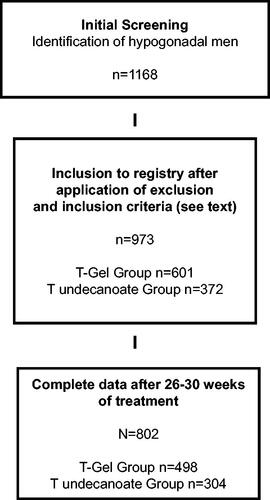
Power calculations were based on the known effects of TRT and retrospective data between treatment forms. Therefore, this power estimation is based on the assumption that hematocrit would increase differentially. We used an estimated effect size of 0.25 with a two-sided α-error = 0.01 and a power = 0.95 and a sample size of at least n = 90 per group was identified. We assumed an attrition rate of 25% (30 men per group). Therefore, each group (T gel vs TU injection) had to include 120 men to start with and 90 men to be available for evaluation at closing of the registry. Post-hoc power analysis using the same parameters on the actual study population (T gel group n = 498, T undecanoate group n = 304, total n = 802) yielded a power = 0.99 (calculated on PASS 14.0). A flow-chart detailing patient selection is presented in .
Evaluation
All other statistical analyses were performed using SPSS (Version 27.0) on data obtained at baseline and between treatment weeks 26–30. Main type of analysis was as follows: Wilcoxon-U-Tests (for independent samples between medication groups, for pairwise tests for change from baseline within medication group). The Shapiro–Wilk test was used for assessment of normal distribution. Variables which were not normally distributed were log-transformed or arcsin-transformed (percentage values) for parametrical analyses. The effect of TRT on hematocrit was evaluated using stepwise analysis of covariance (ANCOVA) for repeated measurements. Stepwise binomial regression models were used to assess triggering a hematocrit of >50%, >52%, >54% or >56% during treatment. Likewise, moving out of anemia range was investigated (increase of hemoglobin of ≥1 g/dl if baseline ≤12.7 g/dl). Levene tests for unequal variances and Breusch–Pagan tests for heteroscedasticity were not significant.
Follow-up of all patients was mandatory and performed between treatment weeks 26–30 for the principal aim and analysis of this registry. Thus, all 802 patients were included with baseline and follow-up visit. Within this cohort and in addition, some patients were also seen before follow-up (83 patients receiving T gel and 34 patients receiving intramuscular TU). Further on, some patients were also checked at additional time points after follow-up for 52 weeks after start of treatment, thus remaining within care of the center for longer than 30 weeks (121 patients receiving T gel and 72 patients receiving intramuscular TU). An analysis of these data points was not part of the initial study plan and results might be biased as these sub-cohorts do not represent the complete group of patients. In an exploratory approach, non-linear logistic regression models for within subject observations over time adjusted for age and waist circumference were calculated to predict the estimated mean increase in hematocrit. This analysis included the above named data points of baseline values and assessments at weeks 26–30 (two times n = 802) but also 310 additional data points (ranging from week 5 to week 52) from 204 patients receiving T gel and 106 patients receiving intramuscular T undecanoate (see above). The fitted models for patients receiving either transdermal T gel or intramuscular T undecanoate were compared by an extra-sum-of-squares F test for putative statistically significant differences. Also here, Levene tests for unequal variances and Breusch–Pagan tests for heteroscedasticity were not significant.
Results
Overall, 802 patients were eligible for data evaluation as per protocol analysis ( for selection process). 375 men had classical forms of hypogonadism (primary form n = 223, secondary form n = 152) and 427 had functional hypogonadism as previously defined [Citation1]. 498 men received T gel and 304 men received T undecanoate.
A graphical display of changes in total T concentrations is given in . displays crude changes in hemoglobin and changes in hematocrit, respectively, as well as significant changes according to Wilcoxon-U-Tests for independent samples between medication groups.
Figure 2. Changes in serum testosterone concentrations.
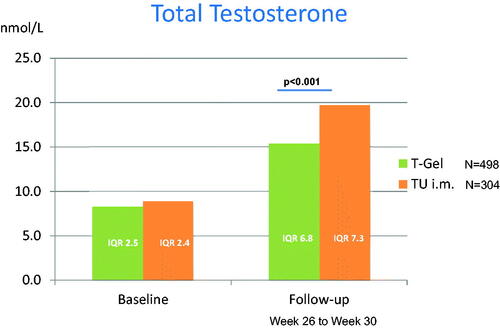
reports baseline values and parameters at follow-up, respectively. There were marked changes from baseline within each group. Patients treated with intramuscular injections of T undecanoate exhibited a higher increase of serum concentrations of T compared to those patients treated with transdermal T gel. Patients receiving injections were more likely to develop a high hematocrit. Albeit, they were also more likely to move out of the anemia range.
Table 1. Crude data for both T-gel and testosterone undecanoate groups for baseline and follow-Up including non-parametric analyses.
displays a stepwise analysis of covariance (ANCOVA) for repeated measurements on general increases of hemoglobin content and hematocrit, corroborating the basic results of and widening them to further parameters of influence on red blood cells: advanced age, a higher delta of testosterone compared to baseline and a higher waist circumference contribute to the risk of increasing the hematocrit.
Table 2. Affectors of increases in hemoglobin and hematocrit during testosterone therapy – ANCOVA models for repeated measurements.
shows the results of stepwise binomial regression models for reaching a hematocrit of >50% and >52% (>54% or >56% were not evaluable) during treatment. The results are in agreement with those reported in . Functional hypogonadism appears as an additional risk factor. Corroborating effects are found regarding elevation of hemoglobin content out of the anemia range and are displayed in .
Table 3. Affectors of reaching a threshold in hematocrit during testosterone therapy: stepwise binomial regression models.
Table 4. Affectors of moving out of anemia range during testosterone therapy: stepwise binomial regression models.
reports the analysis in a subset of patients (n = 574) who received an analysis of the CAG repeat polymorphism of the androgen receptor gene. The results of are supported, longer androgen receptor gene CAG repeats are associated with an attenuated effect on the increase of hemoglobin and hematocrit. Treatment week (26–30) was introduced as covariate, but had no significant effect.
Table 5. Subgroup including all men with genetic analysis of the androgen receptor: Affectors of increases in hemoglobin and hematocrit during testosterone therapy – ANCOVA models for repeated measurements.
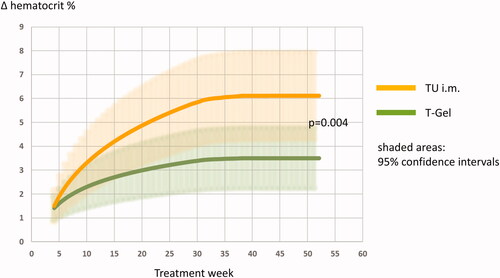
displays non-linear logistic regression models for within subject observations over time as estimates of the mean increase in hematocrit with 95% confidence intervals according to medication using all available data points (2 times 802 plus 310), adjusted for age and waist circumference. The fitted models were significantly different between medication groups by an extra-sum-of-squares F-test (p = 0.004).
Discussion
This registry reports the first direct comparison of a transdermal T gel preparation with the intramuscularly injected long-acting T undecanoate in regard to the effects on hematopoiesis in hypogonadal men within a large real-life registry. Patients receiving T gel are less likely to develop an elevated amount of red blood cells, i.e. an elevated hematocrit than those men receiving the long-acting T undecanoate.
Erythropoiesis is stimulated by T supplementation [Citation16]. In cases when hypogonadal men are anemic, which often results in overall complaints of weakness and fatigue, this effect is considered beneficial [Citation1,Citation3,Citation22]. Notwithstanding, stimulation of erythropoiesis may cause an elevated hematocrit. Hematocrit values over 50–52% can be related to increased blood viscosity and neurological studies report that an elevated hematocrit can result in cerebral ischemia, as a high number of erythrocytes may facilitate platelet activation and aggregation [Citation25–27]. Risk factors in a prospective study involving 1000 stroke patients was, controlling for arterial hypertension, smoking, diabetes mellitus and hypercholesterolemia, a high hematocrit (≥50%) [Citation28]. It remains unclear whether a high hematocrit caused by T treatment has the same relevance as described in the stroke studies, since testosterone exerts direct counterregulatory effects on hemostasis [Citation29]. As long as this has not been clarified, the hematocrit should (for safety reasons) always be regularly monitored in patients receiving a TRT.
Our findings corroborate earlier reports on short-acting testosterone esters compared to transdermal preparations as well as the effect of advancing age on the impact of androgens on hematopoiesis [Citation14,Citation30,Citation31]. Our results also show that, independently from preparation and age, androgen receptor susceptibility, obesity and functional hypogonadism augment the effect of T in hypogonadal men on hematocrit. The effect of T replacement on hematopoiesis obviously reaches a plateau after 26–30 weeks after initiation of treatment (), supporting data from previous reports [Citation3,Citation14].
In this study, there was no T enanthate group. Nevertheless, we report and cite other studies (see above) that compare the short-acting T enanthate to T-gel and T-implants. T enanthate seems to behave similarly to TU, maybe even stronger. Lacking a T enanthate group might be a minor bias of this registry.
The difference between the two T preparations might be explained by T kinetics. As T gel leads to serum concentrations of androgens that mimic a circadian rhythm [Citation32], the long-acting intramuscular testosterone preparation creates permanently high T levels, thus a higher Area Under The Curve for T than the gel [Citation33,Citation34]. We demonstrate that a higher delta T during treatment is a significant contributor to stimulate erythropoiesis.
Likewise, the likelihood for patients to move out of the anemia range seems to be higher for those men receiving intramuscular long-acting T undecanoate but it is possible for T gel as well, albeit they might need a longer time (also see ). This is in agreement with a recent larger trial comparing a transdermal T gel vs. placebo [Citation3].
Figure 3. (a) Changes in hemoglobin. (b) Changes in hematocrit.
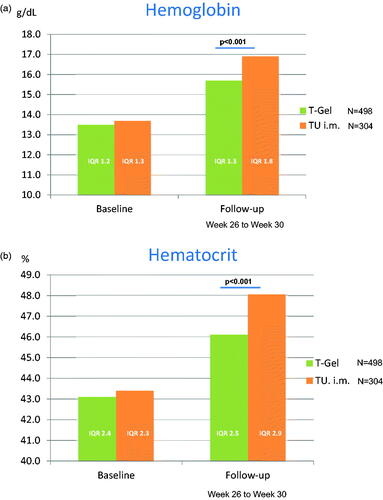
Figure 4. Changes in hematocrit according to regression models.
Albeit older patients were generally likely to develop an elevated hematocrit, those patients who were older and simultaneously anemic had a lesser probability to move out of the anemia range (). So a disctinction between older hypogonadal men as a whole group per se and those who are presenting with hypogonadism and anemia has to be made. While the first group responds more strongly to androgen stimulation in terms of hematopoiesis than younger men, those men with anemia and older age seem not to be as responsive as younger hypogonadal men with anemia.
A recent observational study involving 63 younger and 63 elderly patients receiving intramuscuclar TU over a median period of 12 years observed an elevated hematocrit in 40% of all patients and the effect did not appear to be age-dependent. Albeit, adjustments in terms of prolongation of injection intervals of TU were performed 44% more often in the elderly compared to younger patients and a reason to prolong the injection interval was elevated hematocrit. So the reported effect of age-independence might have been induced by the above named adjustments [Citation35].
Another study reports a median increase of 4–5% in hematocrit in men receiving intramuscular TU vs controls which is in agreement with our findings (). Men with a higher hematocrit than 49% seemed to have a lower mortality than those who did not respond that strongly to the androgen therapy. However, a logistic regression analysis revealed that HCT at final assessment was not associated with mortality (OR: 0.92, 95% CI: 0.81–1.04, p = 0.20) when adjusting for the confounding variables (TT, WC, HbA1c, BP, and lipid values) at final assessment [Citation36]. It may thus be that hematocrit was an indicator of beneficial effects of TRT in terms improving glucose metabolism and lipid profiles. The group had reported before that hematocrit is an essential factor in monitoring a TRT, mentioning that consequences of an increased hematocrit may be mediated by alterations in blood viscosity, oxygen delivery, and flow and that such relative impact may vary in different vascular beds [Citation37].
There are also other factors that have to be observed during testosterone therapy. These are, among others, PSA levels and spermatogenesis. Changes (slight increases) of PSA-levels occurred within the TU group but not in the T-gel group. This is shown in . The difference was statistically different between treatment groups. Nevertheless, PSA levels did not surpass a level of 4.0 ng/ml in any subject. It might be assumed that TU has a stronger effect on prostate tissue than T-gel, corresponding to the findings regarding hematocrit.
Spermatogenesis is usually suppressed by external testosterone administration. It can be assumed that T-gel has a lesser effect also in this regard. Albeit in this cohort, the wish for paternity was not present in any subject as this was an exclusion criterion. Treatment with testosterone would have been contraindicated. Thus we cannot provide data on spermatogenesis, as subjects did not donate semen samples.
Assessment of serum T levels in men receiving with transdermal gels at peak and at minimum could be useful in providing a finely tailored treatment for hypogonadal men, maybe even further reducing the risk of increased hematocrit, both preventing supra-physiological levels and maintaining adequate concentrations through the day. Generally, a paper regarding this reports a stable effect of T levels in men receiving T gels [Citation28].
In summary, special attendance during T treatment seems to be required in obese older men with functional hypogonadism, as adverse effects on hematocrit are more prevalent in this subgroup. Albeit the positive effects of the long-acting intramuscular preparation of T undecanoate on glucose metabolism have recently been described, especially these patients exhibited a high amount of increased hematocrit compared to placebo in that trial [Citation15]. This effect is also visible in the study results reported here. Pharmacogenetics exerted by the androgen receptor are an additional factor tailoring individual T effects.
Overall, both preparations of T are suitable to treat hypogonadal men and move their T levels into the normal range.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Corona G, Goulis DG, Huhtaniemi I, et al. 2European academy of andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European society of endocrinology. Andrology. 2020;8(5):970–987.
- Snyder PJ, Bhasin S, Cunningham GR, et al. Lessons from the testosterone trials. Endocr Rev. 2018;39(3):369–386.
- Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of testosterone levels with anemia in older men: a controlled clinical trial. JAMA Intern Med. 2017;177(4):480–490.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350(5):482–492.
- Kenny AM, Prestwood KM, Gruman CA, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–72.
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(6):1966–1972.
- Sih R, Morley JE, Kaiser FE, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–1667.
- Kapoor D, Goodwin E, Channer KS, et al. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906.
- Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457.
- Aghazadeh M, Pastuszak AW, Johnson WG, et al. Elevated dihydrotestosterone is associated with testosterone induced erythrocytosis. J Urol. 2015;194(1):160–165.
- Fernandez-Balsells MM, Murad MH, Lane M, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–2575.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122.
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92(10):3844–3853.
- Pastuszak AW, Gomez LP, Scovell JM, et al. Comparison of the effects of testosterone gels, injections, and pellets on serum hormones, erythrocytosis, lipids, and prostate-specific antigen. Sex Med. 2015;3(3):165–173.
- Wittert G, Bracken K, Robledo KP, et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): a randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021;9(1):32–45.
- Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6(1):77–85.
- Bachman E, Feng R, Travison T, et al. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab. 2010;95(10):4743–4747.
- Hennigar SR, Berryman CE, Harris MN, et al. Testosterone administration during energy deficit suppresses hepcidin and increases iron availability for erythropoiesis. J Clin Endocrinol Metab. 2020;105(4):dgz316.
- Guo W, Schmidt PJ, Fleming MD, et al. Hepcidin is not essential for mediating testosterone's effects on erythropoiesis. Andrology. 2020;8(1):82–90.
- Bhattacharya RK, Khera M, Blick G, et al. Effect of 12 months of testosterone replacement therapy on metabolic syndrome components in hypogonadal men: data from the Testim registry in the US (TRiUS). BMC Endocr Disord. 2011;11(1):18.
- Rosen RC, Wu FC, Behre HM, et al. Registry of hypogonadism in men (RHYME): design of a multi-national longitudinal, observational registry of exogenous testosterone use in hypogonadal men. Aging Male. 2013;16(1):1–7.
- https://uroweb.org/guideline/male-hypogonadism/.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Zitzmann M, Depenbusch M, Gromoll J, et al. Prostate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic study. J Clin Endocrinol Metab. 2003;88(5):2049–2054.
- Lowe GD. Rheological influences on thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12(3):435–449.
- Lowe GD, Forbes CD. Platelet aggregation, haematocrit, and fibrinogen. Lancet. 1985;325(8425):395–396.
- Wannamethee G, Perry IJ, Shaper AG. Haematocrit, hypertension and risk of stroke. J Intern Med. 1994;235(2):163–168.
- Sansone A, Sansone M, Selleri R, et al. Monitoring testosterone replacement therapy with transdermal gel: when and how? J Endocrinol Invest. 2019;42(12):1491–1496.
- Zitzmann M, Junker R, Kamischke A, et al. Contraceptive steroids influence the hemostatic activation state in healthy men. J Androl. 2002;23:503–511.
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90(2):678–688.
- Dobs AS, Meikle AW, Arver S, et al. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab. 1999;84(10):3469–3478.
- Kaufman JM, Miller MG, Garwin JL, et al. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med. 2011;8(7):2079–2089.
- Eckardstein S, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl. 2002;23(3):419–425.
- Weinbauer GF, Partsch C-J, Zitzmann M, et al. Pharmacokinetics and degree of aromatization rather than total dose of different preparations determine the effects of testosterone: a nonhuman primate study in Macaca fascicularis. J Androl. 2003;24(5):765–774.
- Abildgaard J, Petersen JH, Bang AK, et al. Long-term testosterone undecanoate treatment in the elderly testosterone deficient male: an observational cohort study. Andrology. 2022;10(2):322–332.
- Strange RC, König CS, Adeeba A, et al. Testosterone therapy: Increase in hematocrit is associated with decreased mortality. Androgens. 2021;2(1):150–159.
- König CS, Balabani S, Hackett GI, et al. Testosterone therapy: an assessment of the clinical consequences of changes in hematocrit and blood flow characteristics. Sex Med Rev. 2019;7(4):650–660.