Abstract
Testosterone therapy (TTh) is the primary treatment for aging men with functional hypogonadism. Whilst the benefits of testosterone (T) replacement are well-evidenced, the long-term data for TTh on metabolic and endocrine parameters is limited. Here we present the effect of TTh on endocrine parameters in hypogonadal men at a 12-year follow-up. In this single-centre, cumulative, prospective, registry study, 321 hypogonadal men (mean age: 58.9 years) received testosterone undecanoate injections in 12-week intervals for up to 12 years. Blood samples were taken at every other visit to measure levels of total T (TT), calculated free T, sex hormone-binding globulin (SHBG), estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), progesterone and prolactin. We observed an increase in TT of 15.5 nmol/L (p < 0.0001), a reduction in SHBG of 10.5 nmol/L (p < 0.0001) and an increase in calculated free T of 383.04 pmol/L (p < 0.0001) over the study period. This was accompanied by an increase in estradiol levels by 14.9 pmol/L (p < 0.0001), and decreases in progesterone (0.2 ng/mL, p < 0.0001), LH (10.4 U/L, p < 0.0001) and FSH (8.4 U/L, p < 0.0001) were demonstrated at 12-years. The levels of prolactin remained unchanged. Long-term TTh altered hormonal parameters to predictably modify the endocrine system. These effects were sustained during the entire observation time of 12 years.
Introduction
The age-associated decline in total testosterone (TT) is a well-established phenomenon in men and is termed functional hypogonadism when symptomatic [Citation1–3]. According to the Baltimore Longitudinal Study of Aging (BLSA), nearly 12% of men in their 50s are hypogonadal, which increases to 20% and 30% for those in their 60s and 70s respectively [Citation1]. Increased life expectancy and the rising prevalence of obesity and type II diabetes, especially in western society, are expected to further increase the prevalence of hypogonadism in coming years [Citation4].
Hypogonadism, or T deficiency, is characterised by impaired libido, fatigue, infertility and increased risk of depression and therefore can have a significant impact on quality of life (QoL) [Citation5,Citation6]. Furthermore, an increase in all-cause and cardiovascular mortality consistently correlated with low T in large population studies [Citation7,Citation8]. Metabolic syndrome (MetS) which encompasses obesity and its associated comorbidities such as hypertension, dyslipidemia, insulin resistance, dysregulation of glucose metabolism and decrease in muscle mass also correlates with lower TT levels [Citation9] and thereby contributes to its association to cardiovascular disease.
T deficiency and its associated symptoms are potentially reversible and testosterone therapy (TTh) remains the primary treatment option. Indeed, several long-term studies have reported improved sexual function, body composition and reduced risk of CVD following TTh in hypogonadal men [Citation10–16]. Whilst the immediate beneficial effects of TTh are well evidenced, the long-term effects of exogenous T on physiological parameters remain limited. In a previous report, we described the long-term effects of TTh on anthropometric and metabolic parameters in hypogonadal men at a 10-year follow-up [Citation12], and here we present an update on the endocrine parameters at 12 years in our patient cohort.
Patients and methods
This was a population-based single-centre, prospective, cumulative registry study in 321 hypogonadal men (mean age: 58.9 ± 9.52 years; age-range: 19–84 years) receiving long-acting T undecanoate (TU) (Nebido®, Bayer AG, Berlin, Germany) TTh as 1000 mg injections in 12-week intervals for a maximum of 12 years. The baseline TT in the cohort was ≤3.50 ng/mL (≤12.1 nmol/L), and the patients had at least moderate symptoms of hypogonadism as assessed by the Aging Males’ Symptoms (AMS) scale [Citation17]. Approval from the ethics committee was obtained at the institution in line with guidelines formulated by the German Ärztekammer (German Medical Association). Patients were enrolled after signing informed written consent and all data was treated with confidentiality.
As previously described [Citation11,Citation18], TTh was temporarily interrupted in 147 men after a mean duration of 65.5 months due to reimbursement problems (n = 140), or a diagnosis of prostate cancer (n = 7). The mean duration of this interruption was 16.9 months, and the treatment was resumed thereafter. Three patients dropped out of the study for unknown reasons. Treatment compliance was 100% as all injections were administered at the clinical visit and were documented.
Blood samples were collected from the patients at every other visit throughout the study period. Endocrine parameters consisting of TT, SHBG (sex hormone-binding globulin), estradiol, luteinising hormone (LH), follicle-stimulating hormone (FSH), progesterone and prolactin were measured by an independent commercial laboratory. Free T was calculated using Vermeulen’s formula [Citation19].
Statistical analysis
The data for patients are averaged across each year of their participation in the study and are expressed as mean values with standard deviations at each time point. Baseline parameter values are recorded before the first TU injection. Analysis of variance (ANOVA) was used to compare continuous variable change from baseline and change from the previous year. Statistical analysis was performed using the Statistical Package for Social Sciences v.18 (SPSS Inc., Chicago, USA) and GraphPad Prism version 8.4.3 (GraphPad Software, La Jolla, CA, USA). A value of p < 0.05 was considered significant.
Results
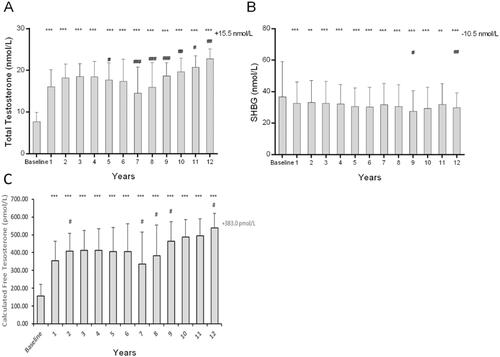
Following TTh, we observed an increase in TT from 7.74 ± 2.14 nmol/L at baseline to within a range of 17 to 23 nmol/L (p < 0.0001), with a mean of 22.83 ± 2.34 nmol/L indicating an average increase of 15.5 nmol/L. Serum TT increased significantly in the first two years of the treatment, after which a steady state was reached (). Furthermore, in the group of patients that had their treatment interrupted, a temporary reduction in baseline levels in TT was observed (data reported elsewhere [Citation18]). From pre-treatment levels of 36.68 ± 22.45 nmol/L, SHBG levels declined steadily upon TU administration to a level of 29.91 ± 9.17 nmol/L at the 12-year follow-up (p < 0.0001; ). Free T significantly increased in the first two years of the treatment, after which a steady state was reached (). Furthermore, in the group of patients that had their treatment interrupted, a temporary reduction in baseline levels in free T was observed (data reported elsewhere [Citation16]).
Figure 1. Three Bar Charts show the effect of long-term effect of TTh on (A) total testosterone, (B) sex hormone binding globulin (SHBG) and (C) calculated free testosterone in hypogonadal men. All data are presented as mean ± SD and the values on the top-right represent the change from baseline at 12 years (*p < 0.05; **p < 0.01 and ***p < 0.0001 vs. baseline and # represents significance vs. previous year).
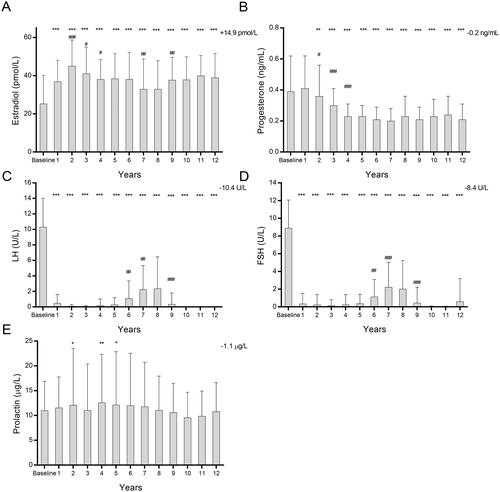
A significant increase in the levels of estradiol was observed at the 12-year follow-up, rising from the baseline of 25.26 ± 14.83 pmol/L to 38.76 ± 13.05 pmol/L (). The most substantial increase occurred in the first two years of treatment after which a plateauing of the estradiol levels was observed for the duration of the study. The serum levels of progesterone, LH and FSH decreased from the baseline levels of 0.39 ± 0.23 ng/mL to 0.23 ± 0.09 ng/mL (p < 0.0001), 10.31 ± 3.71U/L to 0.031 ± 0.02U/L (p < 0.0001) and 8.84 ± 3.17U/L to 0.63 ± 2.59U/L (p < 0.0001) respectively (). The gonadotropins (LH and FSH) increased temporarily in the patient group with interrupted treatment upon cessation of TTh but lowered after the TTh recommenced (data shown elsewhere [Citation18]). The levels of prolactin remain unchanged from a baseline of 11.0 ± 5.86 µg/L to 10.8 ± 5.85 µg/L at a 12-year follow-up ().
Figure 2. Five Bar Charts showing the effect of long-term effect of TTh on (A) estradiol, (B) progesterone, (C) luteinizing hormone (LH), (D) follicle stimulating hormone (FSH) and (E) prolactin in hypogonadal men. All data are presented as mean ± SD and the values on the top-right represent the change from baseline at 12 years (*p < 0.05; **p < 0.01 and ***p < 0.0001 vs. baseline and # represents significance vs. previous year).
Discussion
Declining serum T levels in men are well-documented and TTh is a widely used treatment option to alleviate the physiological symptoms associated with functional hypogonadism [Citation20,Citation21]. To date, there has been limited evidence on the long-term effects of TTh on physiological parameters. Whilst we and others have provided some evidence of sustained benefit of TTh on body mass index, weight circumference and other metabolic parameters glucose metabolism [Citation13,Citation22–25], the long-term effects on the endocrine profile of hypogonadal men remain underexplored. Here, we present data for the effect of TTh on a range of hormones in 321 hypogonadal men at a 12-year follow-up. This is of particular importance as monitoring of endocrine parameters following TTh can inform on the timeline where peak beneficial effects of T are reached and subsequently maintained. Moreover, alterations in the endocrine parameters may also provide an insight into any tolerance or insensitivity that may develop to TTh as reported in some cases [Citation26,Citation27].
We observed a sustained elevation in TT levels over the 12-year period compared to the pre-treatment baseline, consistent with previous long-term studies [Citation12,Citation22]. This demonstrates the efficacy of TU as a treatment modality for T replacement returning levels to within the physiological range for men. Indeed, adherence to treatment was 100% as the administration was controlled and documented in the clinical setting. This along with the long-acting formulation extending interval-between-treatment compared to daily transdermal or nasal applications, make TU an advantageous mode of long-term TTh with sustained effects. Importantly, no major adverse cardiac event occurred during the entire observation time. Concomitant to the increase in TT, there was a decline in SHBG over the study period, therefore resulting in an increase in calculated free T levels. Under normal physiology, approximately 40–65% of the total T is thought to be bound to SHBG and only 1–2% is free T [Citation28]. Although there is increasing evidence to suggest that SHBG can participate in signal transduction via its own receptor [Citation29,Citation30], and that T may be able to signal at least partly via cell surface receptors negating the need to be carrier-free to cross the cell membrane [Citation31], the majority of the physiological role of T is mediated by free T fraction. In fact, free T has been shown to be a stronger predictor of hypogonadism as well as hypertension and risk of prostate cancer [Citation32–34]. Increasing both TT and calculated free T in the present study suggests that the benefits of T action, regardless of signalling mechanism, will be maintained whilst patients receive TTh. Free T levels returned to baseline in the interrupted group further suggesting that long-term TTh is required to maintain the beneficial effects of T supplementation in hypogonadal men.
Although TTh improves the QoL of hypogondal men, aromatisation of T, primarily in adipose tissue, can lead to increases in serum estradiol [Citation35]. The extent to which T signals via conversion to estradiol and subsequent activation of the estrogen receptor to undertake tissue-specific actions remains unknown, but the aromatisation to estrogen is responsible for the majority of negative feedback of T on the hypothalamic-pituitary axis [Citation14]. The increased estradiol levels may also have implications for gynecomastia [Citation36], and alterations in inflammatory and immune responses [Citation37,Citation38]. We did observe a dramatic rise in the serum estradiol in the first two years of the TTh, after which a plateau was reached. Nevertheless, the mean serum estradiol concentrations at 12 years following TTh were measured to be within the reference range of 10–40pg/mL for men as outlined by the Endocrine Society [Citation39,Citation40], and thus may have limited clinical consequences. In our previous investigation, we reported a significant reduction in BMI and waist circumference for 411 hypogonadal men on long-term TTh [Citation23]. This reduction in obesity and consequently adipose tissue might contribute to lower aromatization of T and thus plateauing of estradiol levels within the reference range observed here. However, serum estradiol monitoring in hypogonadal men with TTh may still be useful, especially if clinical symptoms develop, and T dose-titration or use of aromatase inhibitors could be required [Citation36]. Moreover, it has been suggested that instead of the effect of estradiol itself, the ratio of T to estradiol may be more clinically informative [Citation41–43].
Progesterone is a precursor to T and very low levels of the hormone affect spermatogenesis in men, and its metabolites have functions in the central nervous system, specifically regulating sleep and gonadotropin secretion [Citation44,Citation45]. In healthy adult men, the reference levels of progesterone have been reported to be in the range of 0.13–0.97 ng/mL which encompasses the levels observed in this study [Citation46]. Moreover, even though we observe a decrease in progesterone levels over the time course of the study, the magnitude of the effect is small and any biological consequences of this decrease are unlikely.
The hypothalamic-pituitary-testicular (HPT) axis maintains a dynamic equilibrium of serum levels of the reproductive hormones through a closed feedback loop to regulate hypothalamic gonadotropin-releasing hormone (GnRH), LH and FSH produced in the pituitary gland and T in the testis. TTh and the resultant increase in serum TT levels have been shown to suppress LH and FSH levels previously [Citation47], and in agreement with this study. Another report suggests that the suppression of LH secretion during TTh is greater as men age due to alterations in the negative feedback regulation of GnRH via increased estradiol [Citation48]. The increased estradiol observed in this study may therefore mediate a similar feedback regulation of gonadotropins thereby suppressing LH and FSH levels. TTh mediated suppression of LH and FSH could lead to a decrease in semen parameters and infertility, especially with longer-acting T formulations. Indeed, a study has suggested that shorter-acting T with daily troughs between doses, may allow for a more physiological relevant pulsatile release of GnRH that maintains the production of LH and FSH [Citation49]. Nevertheless, a well-designed clinical trial is necessary to determine whether short-acting TTh improves the symptoms associated with hypogonadism.
Whilst prolactin receptors are present in the male reproductive organs, the exact role of this hormone in male reproductive physiology remains unclear [Citation50]. The secretion of GnRH by the hypothalamus is pulsatile in nature and triggers LH and consequently T synthesis. Prolactin inhibits this pulsatile secretion of GnRH and therefore the release of LH and T. Serum prolactin did not alter during the study period and remained within the reference range of 2–18ng/mL for males [Citation51]. This is of importance as even mild to moderate increases in serum prolactin, if chronic, may negatively affect spermatogenesis and fertility as suggested by studies in adult rats [Citation52]. This effect on fertility may be due to its participation in the HPT axis described above [Citation53], or via reduction in androgen-binding protein expression which is a paracrine regulator of spermatogenesis as described by Aleem et al. [Citation52].
The present study has limitations. This was an observational study with no placebo-controlled group and thus does not allow direct effects of treatment versus non-treatment to be compared therefore limiting the scope of interpretation of the presented findings. Furthermore, a cohort of 147 patients had TTh interrupted due to reimbursement issues and/or diagnosis of prostate cancer. Whilst this may skew the data presented here, especially for years 6–8, we have observed both here and previously [Citation11], that with the recommencement of TTh the hormone concentrations return to pre-interruption levels. Thus, it is unlikely that the interruption affected the circulating levels of the hormones measured at the final 12-year follow-up.
In conclusion, this study provides comprehensive data on the long-term effects of TTh on endocrine parameters in hypogonadal mainly elderly men. The data suggest that TTh affects the hormonal parameters as expected by increasing TT, increasing estradiol but within the reference range, suppressing LH and FSH as part of the HPG feedback loop, decreasing progesterone and having no effect on prolactin. Importantly, these effects are sustained during the entire observation time of 12 years.
Acknowledgements
Open Access funding is provided by the Qatar National Library. Editorial support for this manuscript was provided by Astra-Health, www.Astra-Health.com
Disclosure statement
AY received partial compensation for data entry, honoraria and occasionally travel grants from Bayer AG. Member of Advisory Board for Testosterone, Besins Health Care, Pharma.
References
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86(2):724–731.
- Morley JE, Kaiser FE, Perry HM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413.
- Snyder PJ. Effects of age on testicular function and consequences of testosterone treatment. J Clin Endocrinol Metab. 2001;86(6):2369–2372.
- Seftel AD. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res. 2006;18(2):115–120.
- Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the rancho Bernardo study. J Clin Endocrinol Metab. 1999;84(2):573–577.
- Monteagudo PT, Falcão AA, Verreschi ITN, et al. The imbalance of sex-hormones related to depressive symptoms in obese men. Aging Male. 2016;19(1):20–26.
- Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007–3019.
- Kato Y, Shigehara K, Inaba T, et al. Low free testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Aging Male. 2021;24(1):8–14.
- Boden WE, Miller MG, McBride R, et al. Testosterone concentrations and risk of cardiovascular events in androgen-deficient men with atherosclerotic cardiovascular disease. Am Heart J. 2020;224:65–76.
- Permpongkosol S, Khupulsup K, Leelaphiwat S, et al. Effects of 8-year treatment of long-acting testosterone undecanoate on metabolic parameters, urinary symptoms, bone mineral density, and sexual function in men with late-onset hypogonadism. J Sex Med. 2016;13(8):1199–1211.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19(1):64–69.
- Yassin AA, Nettleship J, Almehmadi Y, et al. Effects of continuous long-term testosterone therapy (TTh) on anthropometric, endocrine and metabolic parameters for up to 10 years in 115 hypogonadal elderly men: real-life experience from an observational registry study. Andrologia. 2016;48(7):793–799.
- Yassin DJ, Doros G, Hammerer PG, et al. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–1576.
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25–45.
- Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47–71.
- Kato Y, Shigehara K, Nakashima K, et al. The five-year effects of testosterone replacement therapy on lipid profile and glucose tolerance among hypogonadal men in Japan: a case control study. Aging Male. 2020;23(1):23–28.
- Heinemann LA, Saad F, Zimmermann T, et al. The aging males' symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15.
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol. 2016;84(1):107–114.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672.
- Haider A, Yassin A, Doros G, et al. Effects of long-term testosterone therapy on patients with "diabesity": results of observational studies of pooled analyses in obese hypogonadal men with type 2 diabetes. Int J Endocrinol. 2014;2014:1–15.
- Traish AM, Haider A, Doros G, et al. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314–329.
- Saad F, Doros G, Haider KS, et al. Differential effects of 11 years of long-term injectable testosterone undecanoate therapy on anthropometric and metabolic parameters in hypogonadal men with normal weight, overweight and obesity in comparison with untreated controls: real-world data from a controlled registry study. Int J Obes. 2020;44(6):1264–1278.
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes. 2016;40(1):162–170.
- Mathur A, Malkin C, Saeed B, et al. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur J Endocrinol. 2009;161(3):443–449.
- Hajjar RR, Kaiser FE, Morley JE. Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab. 1997;82(11):3793–3796.
- Brabrand S, Fossa SD, Cvancarova M, et al. Androgen substitution with testosterone undecanoate in survivors of bilateral testicular cancer requires individually-adjusted injection intervals. BJU Int. 2011;107(7):1080–1087.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310–320.
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 1981;53(1):58–68.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122(5):751–762.
- Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120.
- Estrada M, Espinosa A, Muller M, et al. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144(8):3586–3597.
- Anawalt BD, Hotaling JM, Walsh TJ, et al. Performance of total testosterone measurement to predict free testosterone for the biochemical evaluation of male hypogonadism. J Urol. 2012;187(4):1369–1373.
- Yang Q, Li Z, Li W, et al. Association of total testosterone, free testosterone, bioavailable testosterone, sex hormone-binding globulin, and hypertension. Medicine. 2019;98(20):e15628.
- Pierorazio PM, Ferrucci L, Kettermann A, et al. Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore longitudinal study of aging. BJU Int. 2010;105(6):824–829.
- Schiffer L, Kempegowda P, Arlt W, et al. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol. 2017;177(3):R125–R143.
- Rhoden EL, Morgentaler A. Treatment of testosterone-induced gynecomastia with the aromatase inhibitor, anastrozole. Int J Impot Res. 2004;16(1):95–97.
- Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6(3):437–451.
- Tsilidis KK, Rohrmann S, McGlynn KA, et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. 2013;1(6):919–928.
- Stanczyk FZ, Clarke NJ. Measurement of estradiol–challenges ahead. J Clin Endocrinol Metab. 2014;99(1):56–58.
- Rosner W, Hankinson SE, Sluss PM, et al. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98(4):1376–1387.
- Zheng HY, Li Y, Dai W, et al. Imbalance of testosterone/estradiol promotes male CHD development. Biomed Mater Eng. 2012;22(1-3):179–185.
- van Koeverden ID, de Bakker M, Haitjema S, et al. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. 2019;115(2):453–462.
- Choi JW, Ryoo IW, Hong JY, et al. Clinical impact of estradiol/testosterone ratio in patients with acute ischemic stroke. BMC Neurol. 2021;21(1):91.
- Oettel M, Mukhopadhyay AK. Progesterone: the forgotten hormone in men? Aging Male. 2004;7(3):236–257.
- Ghoumari AM, Abi Ghanem C, Asbelaoui N, et al. Roles of progesterone, testosterone and their nuclear receptors in central nervous system myelination and remyelination. IJMS. 2020;21(9):3163.
- Burtis CA, Ashwood ER, eds. Tietz Textbook of Clinical Chemistry. Philadelphia: WB Saunders Co, 1999.
- Shimon I, Lubina A, Gorfine M, et al. Feedback inhibition of gonadotropins by testosterone in men with hypogonadotropic hypogonadism: comparison to the intact pituitary-testicular axis in primary hypogonadism. J Androl. 2006;27(3):358–364.
- Winters SJ, Wang C. Fortigel study G. LH and non-SHBG testosterone and estradiol levels during testosterone replacement of hypogonadal men: further evidence that steroid negative feedback increases as men grow older. J Androl. 2010;31(3):281–287.
- Masterson TA, Turner D, Vo D, et al. The effect of longer-acting vs shorter-acting testosterone therapy on follicle stimulating hormone and luteinizing hormone. Sex Med Rev. 2021;9(1):143–148.
- Hair WM, Gubbay O, Jabbour HN, et al. Prolactin receptor expression in human testis and accessory tissues: localization and function. Mol Hum Reprod. 2002;8(7):606–611.
- Al-Chalabi M, Bass AN, Alsalman I. Physiology, prolactin. Treasure Island (FL): StatPearls; 2022.
- Aleem M, Choudhari J, Padwal V, et al. Hyperprolactinemia affects spermiogenesis in adult male rats. J Endocrinol Invest. 2005;28(1):39–48.
- Gill-Sharma MK. Prolactin and male fertility: the long and short feedback regulation. Int J Endocrinol. 2009;2009:687259.
