Abstract
Objectives
To investigate the association between oxidative stress and erectile dysfunction (ED) in community-dwelling men and men on dialysis.
Methods
This cross-sectional study included 398 community-dwelling men and 42 men on dialysis. Oxidative stress was assessed using 8-hydroxy-2'-deoxyguanosine (8-OHdG). Univariable and multivariable logistic regression analyses were performed to evaluate the association between oxidative stress and ED.
Results
Spearman’s rank correlation test showed no significant correlation between urine 8-OHdG levels and the 5-Item International Index of Erectile Function scores in community-dwelling men (ρ = −0.005, p = 0.917) and between plasma 8-OHdG levels and the Sexual Health Inventory for Men scores in men on dialysis (ρ = 0.166, p = 0.295). In community-dwelling men, univariable and multivariable analyses revealed that urine 8-OHdG level was not significantly associated with ED (odds ratio [OR]: 1.005, 95% confidence interval [CI]: 0.884–1.144, p = 0.934; OR: 0.930, 95% CI: 0.798–1.084, p = 0.353; respectively). In men on dialysis, univariable analyses revealed that plasma 8-OHdG level was not significantly associated with severe ED (OR: 0.967, 95% CI: 0.876–1.066, p = 0.498).
Conclusions
Oxidative stress was not significantly associated with ED prevalence and severity in community-dwelling men and men on dialysis.
Introduction
Erectile dysfunction (ED) is defined as the inability to achieve or maintain sufficient penile erection for satisfactory sexual intercourse and frequently perceived by the patient as a serious condition [Citation1,Citation2]. It has been reported that the global prevalence of ED was 3.0–77% [Citation3].
Oxidative stress reflects an imbalance between the production of reactive oxygen species (ROS) and host antioxidant defense systems [Citation4]. Oxidative stress decreases the bioavailability of nitric oxide (NO) and induces mitochondrial dysfunction, inflammation, and endothelial NO synthase uncoupling. These develop endothelial dysfunction by increasing the adhesion of monocytes to endothelial cells (ECs), impairing the angiogenic potential of ECs, and inducing apoptosis. In addition, ROS-induced mitochondrial dysfunction contributes to the additional ROS production by altered mitochondrial metabolism, leading to the exacerbation of endothelial dysfunction. Endothelial dysfunction enhances the phenotypic change of vascular smooth muscle cells and eventually contributes to the development of atherosclerotic disease and cardiovascular disease (CVD) [Citation5].
The preceding evidences suggest that oxidative stress is closely associated with well-known risk factors of ED, including hypertension (HTN), dyslipidemia, diabetes mellitus (DM), metabolic syndrome, and hypogonadism [Citation6–10]. Therefore, numerous studies have investigated the role of oxidative stress in the etiology of ED using animal models [Citation11–14] and identified oxidative stress as a potential therapeutic target. However, the association between oxidative stress and ED in humans remains to be elucidated. Thus, we aimed to evaluate this association in community-dwelling men in the present study.
Moreover, although it has been reported that end-stage renal disease (ESRD) and dialysis were associated with elevated oxidative stress [Citation15–18], no study has investigated the association between oxidative stress and ED in patients with these backgrounds. We hypothesized that oxidative stress might contribute greatly to the severity of ED in men on dialysis. Therefore, we also aimed to evaluate this association in men on dialysis.
Materials and methods
Ethics statement
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. This study was comprised of two cross-sectional studies that evaluated community-dwelling men and men on dialysis. The data of community-dwelling men were collected from the Iwaki Health Promotion Project, and the study was approved by the ethics review board of Hirosaki University Graduate School of Medicine (authorization number: 2014–377). The project was designed to prevent lifestyle-related diseases, promote health, and extend the lifespan of the residents of Hirosaki City (Iwaki District) in collaboration with Hirosaki City, Hirosaki University, and the Aomori Prefecture General Screening Centre. All the participants voluntarily participated in the project and provided written informed consent. The other cross-sectional study evaluating men on dialysis was approved by the ethics review board of Mutsu General Hospital (authorization number: H28-2). All the participants provided written informed consent.
Participant selection
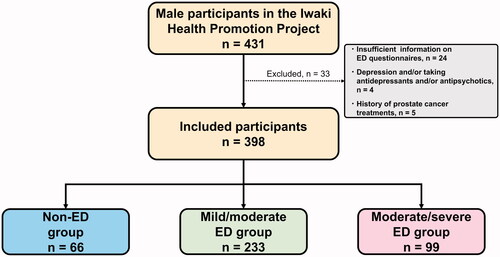
In a community-based cross-sectional study, of the 1,113 participants in the Iwaki Health Promotion Project 2015, 431 men were selected, excluding 682 women. Of these, 33 were excluded based on the following criteria: (1) insufficient information on ED questionnaires, (2) depression and/or taking antidepressants and/or antipsychotics, and (3) history of prostate cancer treatments, such as radical prostatectomy, radiation therapy, and androgen-deprivation therapy, because these therapies affect erectile function [Citation19,Citation20]. In total, 398 community-dwelling men were included in the analysis ().
Figure 1. Participant selection of community-dwelling men. The number of included and excluded participants is shown. ED: erectile dysfunction.
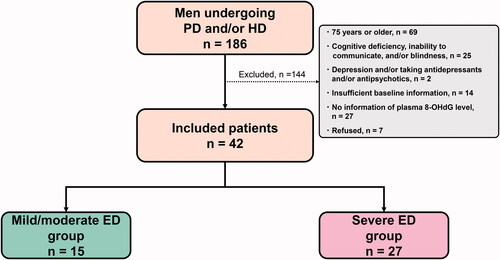
The other cross-sectional study assessed 186 men undergoing peritoneal dialysis (PD) and/or hemodialysis (HD) between July 2016 and May 2018 at Mutsu General Hospital. Of the 186 men, 144 were excluded based on the following exclusion criteria: (1) age ≥75 years; (2) cognitive deficiency, blindness, and/or inability to communicate; (3) depression and/or taking antidepressants and/or antipsychotics; (4) insufficient baseline information, including plasma 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels; and (5) refusal to participate in this study. Finally, 42 men on PD and/or HD were included in the analysis ().
Figure 2. Participant selection of men on dialysis. The number of included and excluded patients is shown. PD: peritoneal dialysis; HD: hemodialysis; ED: erectile dysfunction; 8-OHdG: 8-hydroxy-2'-deoxyguanosine.
In this study, the sample size was not calculated to include as many participants as possible considering the relatively smaller sample size. Although hypogonadism is one of the most important factors of ED, men with hypogonadism were not excluded considering the limited association between plasma testosterone levels and the prevalence and severity of ED in this study.
Evaluation of variables
The following variables were analyzed: age, body mass index, HTN, dyslipidemia, DM, CVD, mental health status, depression, laboratory values, education level, smoking status, current habitual drinking, and medications being taken (antidiabetic medications, β-blockers, calcium-blockers, thiazide diuretics, spironolactone, and antidepressants). Blood samples were collected in the morning and separated by centrifugation. The laboratory values included creatinine, albumin, calcium, inorganic phosphorus, intact parathyroid hormone, total cholesterol, low-density lipoprotein, and triglycerides and were assayed with standard laboratory techniques. Serum total testosterone was measured using chemiluminescent immunoassay. Payne’s formula was used to calculate the adjusted calcium levels [Citation21]. Hypogonadism was defined as serum total testosterone level ≤300 ng/dL [Citation22]. In community-dwelling men, mental health status was evaluated using the 36-Item Short Form Health Survey Mental Component Summary. Scores were added and transformed into 0–100 scales, with higher values indicating better health status [Citation23]. In men on dialysis, self-reported depression was assessed using the Vitality Questionnaire of the Short Form-36 Health Survey. Patients with certain responses to the questionnaire (i.e. “all of the time” or “most of the time”) and/or who were taking antidepressants were considered to have depression [Citation24]. Smoking status was quantified using the Brinkman index.
Evaluation of ED
In community-dwelling men, ED was assessed using the 5-Item International Index of Erectile Function (IIEF-5) [Citation25]. The IIEF-5 scores were interpreted as follows: no ED (22–25), mild ED (17–21), mild-to-moderate ED (12–16), moderate ED (8–11), and severe ED (5–7). Based on this, participants were divided into the non-ED (IIEF-5 score ≥22), mild/moderate ED (IIEF-5 score 12–21), and moderate/severe ED (IIEF-5 score ≤11) groups ().
In men on dialysis, ED was assessed using the Sexual Health Inventory for Men (SHIM), a validated abbreviated version of the IIEF [Citation26]. The SHIM scores were interpreted as follows: no ED (22–25), mild ED (17–21), moderate ED (8–16), and severe ED (1–7). Based on this, patients were divided into the mild/moderate (SHIM score ≥8) and severe ED (SHIM score ≤7) groups ().
Evaluation of oxidative stress
We used 8-OHdG as a marker of oxidative stress. Among all the bases in nucleic acids, guanine is the most susceptible to oxidative damage and is oxidized to 8-OHdG. Because it is stable and excreted in bodily fluids with DNA repair, 8-OHdG is one of the most widely used and reliable oxidative stress markers [Citation27].
In community-dwelling men, urine samples were used to measure 8-OHdG because urine has been regarded as a preferred diagnostic biofluid considering its sterility, simplicity to obtain large volumes, and noninvasive. Urine creatinine-adjusted urine 8-OHdG levels (ng/mgCr) were measured using the New 8-OHdG Check ELISA kit (Japan Institute for the Control of Aging, Shizuoka, Japan) according to the manufacturer’s instructions. Urine samples were collected in the morning and stored at −80 °C until analysis.
In men on dialysis, plasma samples were used to measure 8-OHdG because they could not provide urine samples. Plasma 8-OHdG levels (ng/ml) were measured using the OxiSelect Oxidative DNA Damage ELISA Kit (Cell Biolabs Inc., San Diego, USA) according to the manufacturer’s instructions. Plasma samples were collected in the morning and stored at −80 °C until analysis.
Statistical analysis
SPSS version 24.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 5.03 (GraphPad Software, San Diego, USA) were used to perform statistical analyses. The Kolmogorov–Smirnov test was used to determine the distribution of the data. Quantitative variables are expressed as medians with interquartile ranges. Categorical variables were compared using the Fisher’s exact test or chi-squared test. Differences in quantitative variables between the two groups and among the three groups were analyzed using the Mann–Whitney U and Kruskal–Wallis tests, respectively. Correlations between variables were analyzed using Spearman’s rank correlation coefficient. Univariable and multivariable logistic regression analyses were performed to evaluate the association between oxidative stress and ED. Statistical significance was set at p < 0.05.
Results
Characteristics of the community-dwelling men
The median age of the community-dwelling men was 53 years. The median IIEF-5 score was 17. Of the 398 men, 66 (17%), 233 (59%), and 99 (25%) were classified into the non-ED, mild/moderate ED, and moderate/severe ED groups, respectively ().
Variables such as age, prevalence of comorbidity, mental health status, renal function, plasma albumin levels, smoking status, and education levels were significantly different among the three groups ().
Table 1. Characteristics of community-dwelling men.
Characteristics of the men on dialysis
The median age and dialysis duration of the men on dialysis were 64 years and 64 months, respectively. Of the 42 patients, 3 (7.1%), 33 (86%), and 3 (7.1%) were undergoing PD, HD, and a combination of once-weekly HD with PD, respectively ().
Table 2. Characteristics of men on dialysis.
The median SHIM score was 4.5. All patients (100%) had any level of ED. Of these, 15 (36%) and 27 (64%) patients were classified into mild/moderate and severe ED groups, respectively (). No patient was receiving any type of ED treatment during the investigation.
No significant differences in the patient background were observed between the two groups, except for the duration of dialysis and plasma phosphorus and intact parathyroid hormone levels ().
Correlations between 8-OHdG levels and ED questionnaire scores
In community-dwelling men, spearman’s rank correlation test showed no significant correlation between urine 8-OHdG levels and IIEF-5 scores (; ρ= −0.005, p = 0.917).
Figure 3. Associations between urine and plasma 8-hydroxy-2'-deoxyguanosine (8-OHdG) levels and the severity of erectile dysfunction (ED). Correlations between urine 8-OHdG level and 5-Item International Index of Erectile Function (IIEF-5) score in community-dwelling men (A) and between plasma 8-OHdG level and Sexual Health Inventory for Men (SHIM) score in men on dialysis (B) were analyzed using Spearman’s rank correlation coefficient. Urine 8-OHdG levels were compared between the three groups using the Kruskal–Wallis test (C). Plasma 8-OHdG levels were compared between the two groups using the Mann–Whitney U test (E). The distribution of the ED degree in community-dwelling men (D) and the prevalence of severe ED in men on dialysis (F) were compared between men with lower and higher 8-OHdG levels using the chi-squared test.
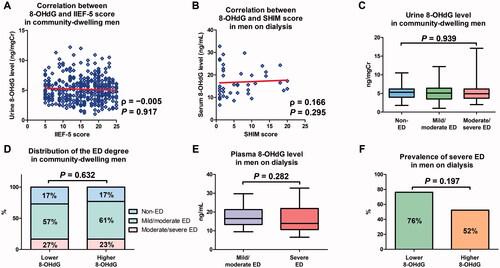
Similarly, Spearman’s rank correlation test showed no significant correlation between plasma 8-OHdG levels and SHIM scores in men on dialysis (; ρ = 0.166, p = 0.295).
Association between 8-OHdG levels and the severity of ED
In community-dwelling men, the median urine 8-OHdG level was 5.0 ng/mgCr. No significant difference in urine 8-OHdG levels was observed among the three groups (; p = 0.939). When these men were divided according to their median urine 8-OHdG values, no significant difference in the distribution of ED degree was observed between men with higher and lower 8-OHdG levels (; p = 0.632).
In men on dialysis, the median plasma 8-OHdG level was 16 ng/mL. No significant difference in plasma 8-OHdG levels was observed between the two groups (; p = 0.282). When these men were divided according to their median plasma 8-OHdG values, no significant difference in the prevalence of severe ED was observed between men with higher and lower 8-OHdG levels (; p = 0.197).
Univariable and multivariable analyses for ED
In community-dwelling men, univariable analyses revealed that urine 8-OHdG levels were not significantly associated with ED (; odds ratio [OR]: 1.005, 95% confidence interval [CI]: 0.884–1.144, p = 0.934). Similarly, in the multivariable analysis, urine 8-OHdG levels were not significantly associated with ED (; OR: 0.930, 95% CI: 0.798–1.084, p = 0.353). When we focused only on men with ED (n = 332), urine 8-OHdG levels were not significantly associated with moderate/severe ED in the univariable analysis (; OR: 1.030, 95% CI: 0.922–1.149, p = 0.604).
Table 3. Univariable and multivariable analyses for erectile dysfunction in community-dwelling men.
Table 4. Univariable analyses for moderate/severe erectile dysfunction in community-dwelling men with erectile dysfunction.
In men on dialysis, univariable analyses revealed that plasma 8-OHdG levels were not significantly associated with severe ED (; OR: 0.967, 95% CI: 0.876–1.066, p = 0.498).
Table 5. Univariable analyses for severe erectile dysfunction in men on dialysis.
Discussion
To the best of our knowledge, this is the first study to investigate the association between urine and plasma 8-OHdG levels and ED in both community-dwelling men and men on dialysis. The results of the present study revealed that urine 8-OHdG levels were not significantly associated with the prevalence of ED in community-dwelling men (). Moreover, urine and plasma 8-OHdG levels were not significantly associated with ED severity in both community-dwelling men and men on dialysis, respectively ( and ). Although the etiology of ED is well-known to be multifactorial [Citation28], these results suggest that oxidative stress may not have an important contribution to the development and severity of ED in humans. Because the present study was a cross-sectional study and did not have an enough sample size, our future prospective study with an enough sample size will clarity the association between oxidative stress and ED in humans.
The present study showed no significant association between urine 8-OHdG levels and the prevalence and severity of ED in community-dwelling men. Although oxidative stress is believed to play an important role in the etiology of ED, this concept may be derived from several basic studies using animal models [Citation11–14]. In humans, a few studies have investigated the association between oxidative stress and ED, but the results have been inconsistent. Aldemir et al. have compared the serum oxidative and antioxidative status between healthy volunteers and patients with ED and showed increased oxidative and decreased antioxidative parameters in patients with ED [Citation29]. Similarly, Yasuda et al. have demonstrated a significant association between salivary 8-OHdG levels and ED severity in healthy volunteers [Citation30]. In addition, Ciftci et al. have revealed that the activity of serum paraoxonase 1, an antioxidant enzyme, in patients with ED was significantly lower than that in healthy men [Citation31]. Although these results suggest a possible contribution of oxidative stress to the etiology of ED in humans, definitive conclusions cannot be drawn because of the small sample size and lack of adjustment for potential confounding variables. In contrast, Yang et al. have conducted a prospective study with a sufficient sample size of men (n = 917) and demonstrated that plasma fluorescent oxidation products, a global biomarker for oxidative stress, were not associated with the development of ED in a multivariable adjusted model [Citation32]. This result was consistent with our findings. Considering the sample size and statistical methods, the concept that oxidative stress does not contribute to the etiology of ED in humans may be predominant. Further studies are required to clarify the association between oxidative stress and ED.
Although we hypothesized that oxidative stress might contribute greatly to the severity of ED in men on dialysis, considering the significant associations of ESRD and dialysis with elevated oxidative stress [Citation15–18], the present study revealed that plasma 8-OHdG levels were not correlated with ED severity at all. The reason for this remains unclear. Because the etiology of ED in men on dialysis is multifactorial [Citation33], oxidative stress alone may be insufficient to develop severe ED. Alternatively, plasma 8-OHdG levels may not accurately reflect oxidative stress in the penile region. Yeni et al. have compared total peroxide levels, an oxidative stress marker, between peripheral venous blood and the penile corpus cavernosum in healthy men and demonstrated no significant correlation between them [Citation34]. Moreover, because 8-OHdG estimates oxidative DNA damage, it cannot reflect oxidative stress generated from other sources, including lipids and proteins [Citation27]. Thus, a single oxidative stress marker may be insufficient for evaluating oxidative stress generated from multiple sources. Contrary to our expectations, the present study was not able to determine possible associations between oxidative stress and ED severity. However, we still cannot make a definitive conclusion, as this cross-sectional study in men on dialysis should be regarded as a preliminary study considering its sample size. In addition, no previous study has investigated the association between oxidative stress and ED in men on dialysis or in an animal model of ESRD. Further studies are warranted to clarify the role of oxidative stress in ED severity in men on dialysis.
This study has several limitations. First, its cross-sectional nature prevents us from determining cause-and-effect associations, and it also may involve selection bias and other unmeasurable confounding factors that we could not control. Second, a relatively small number of men on dialysis was included in this study. Third, we did not have the information on the duration of DM. Lastly, the measurement of 8-OHdG at a single point in time may not accurately reflect the average levels over a prolonged period. Despite these limitations, the results of the present study challenge the traditional understanding on the role of oxidative stress in the etiology of ED in humans.
Conclusions
Oxidative stress was not significantly associated with the prevalence and severity of ED in community-dwelling men and men on dialysis. Considering several limitations in the present study, further studies are warranted to clarify the role of oxidative stress in ED.
Acknowledgments
The authors would like to thank all participants and the data collection team of the Iwaki Health Promotion Project 2015 and the staff of the Department of Social Medicine, Hirosaki University for their contribution to this study. The authors also thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- NIH Consensus Conference. Impotence. NIH consensus development panel on impotence. JAMA. 1993;270:83–90.
- Isidori AM, Giammusso B, Corona G, et al. Diagnostic and therapeutic workup of erectile dysfunction: results from a Delphi consensus of andrology experts. Sex Med. 2019;7:292–302.
- Kessler A, Sollie S, Challacombe B, et al. The global prevalence of erectile dysfunction: a review. BJU Int. 2019;124(4):587–599.
- Collin F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. IJMS. 2019;20(10):2407.
- Shaito A, Aramouni K, Assaf R, et al. Oxidative Stress-Induced endothelial dysfunction in cardiovascular diseases. Front Biosci. 2022;27(3):105.
- Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–667.
- Jabarpour M, Rashtchizadeh N, Argani H, et al. The impact of dyslipidemia and oxidative stress on vasoactive mediators in patients with renal dysfunction. Int Urol Nephrol. 2019;51(12):2235–2242.
- Defeudis G, Mazzilli R, Tenuta M, et al. Erectile dysfunction and diabetes: a melting pot of circumstances and treatments. Diabetes Metab Res Rev. 2022;38:e3494.
- Rani V, Deep G, Singh RK, et al. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193.
- Leisegang K, Roychoudhury S, Slama P, et al. The mechanisms and management of age-related oxidative stress in male hypogonadism associated with non-communicable chronic disease. Antioxidants. 2021;10(11):1834.
- Shukla N, Rossoni G, Hotston M, et al. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103(11):1522–1529.
- Chen Y, Zhou B, Yu Z, et al. Baicalein alleviates erectile dysfunction associated with Streptozotocin-Induced type I diabetes by ameliorating endothelial nitric oxide synthase dysfunction, inhibiting oxidative stress and fibrosis. J Sex Med. 2020;17:1434–1447.
- Wu XJ, Shen WH, He P, et al. Telomerase reverse transcriptase genetically modified adipose tissue derived stem cells improves erectile dysfunction by inhibiting oxidative stress and enhancing proliferation in rat model. Biomed Pharmacother. 2017;92:595–605.
- Muniz JJ, Leite LN, De Martinis BS, et al. Chronic ethanol consumption induces erectile dysfunction: role of oxidative stress. Life Sci. 2015;141:44–53.
- Huang M, Zheng L, Xu H, et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J Mol Cell Cardiol. 2020;138:256–268.
- Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48(5):752–760.
- Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–1016.
- Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–1538.
- Gaither TW, Awad MA, Osterberg EC, et al. The natural history of erectile dysfunction after prostatic radiotherapy: a systematic review and meta-analysis. J Sex Med. 2017;14(9):1071–1078.
- Candy B, Jones L, Williams R, et al. Phosphodiesterase type 5 inhibitors in the management of erectile dysfunction secondary to treatments for prostate cancer: findings from a Cochrane systematic review. BJU Int. 2008;102(4):426–431.
- Payne RB, Little AJ, Williams RB, et al. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4(5893):643–646.
- Marks LS, Mazer NA, Mostaghel E, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296(19):2351–2361.
- Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306(6890):1437–1440.
- Soma O, Hatakeyama S, Imai A, et al. Relationship between frailty and lower urinary tract symptoms among community-dwelling adults. Low Urin Tract Symptoms. 2020;12(2):128–136.
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the international index of erectile function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326.
- Alwaal A, Awad M, Boggs N, et al. Sexual health inventory for men questionnaire as a screening method for erectile dysfunction in a general urology clinic. Sex Med. 2020;8(4):660–663.
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2' -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–139.
- Sivaratnam L, Selimin DS, Abd Ghani SR, et al. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2021;18(1):121–143.
- Aldemir M, Okulu E, Neşelioğlu S, et al. Evaluation of serum oxidative and antioxidative status in patients with erectile dysfunction. Andrologia. 2012;44(Suppl 1):266–271.
- Yasuda M, Ide H, Furuya K, et al. Salivary 8-OHdG: a useful biomarker for predicting severe ED and hypogonadism. J Sex Med. 2008;5(6):1482–1491.
- Ciftci H, Yeni E, Savas M, et al. Paraoxonase activity in patients with erectile dysfunction. Int J Impot Res. 2007;19(5):517–520.
- Yang S, Giovannucci E, Bracken B, et al. Association between plasma fluorescent oxidation products and erectile dysfunction: a prospective study. BMC Urol. 2015;15:85.
- El-Assmy A. Erectile dysfunction in hemodialysis: a systematic review. World J Nephrol. 2012;1(6):160–165.
- Yeni E, Gulum M, Selek S, et al. Comparison of oxidative/antioxidative status of penile corpus cavernosum blood and peripheral venous blood. Int J Impot Res. 2005;17(1):19–22.
