Abstract
Lower urinary tract symptoms (LUTS) are caused by higher tension at the bladder neck level (due to fibrosis or stiffness) or benign prostatic hyperplasia, which causes static obstruction of the bladder outlet. Both forms cause a group of symptoms such as hesitancy, intermittency, weak stream, nocturia, urine frequency, and urgency. Additionally, LUTS (obstructive or irritative symptoms) are common in elderly men with hypogonadism, identified as the reduced testes capability in producing sex steroids and sperm, and are categorized as testosterone deficiency. Even though the mode of action (MoA) of testosterone therapy (TTh) on hypogonadal men needs more researched and understanding, the effectiveness of TTh in the development of male genital organs has been reported in several studies. This review shows the latest updates of TTh in LUTS including potential adverse effects, advantages, and disadvantages.
Graphical Abstract
Keywords:
Introduction
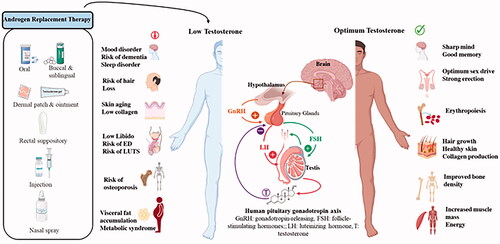
The earth’s population is indeed aging, and there are many age-related health concerns in men have become obvious over the last two decades. One of the current attracting awareness is the significant incidence of symptoms in aging males with hypogonadism such as weariness, loss of libido, lack of physical strength, erectile dysfunction (ED), depression, and visceral obesity, which are all known to be caused by testosterone deficiency (TD) [Citation1–4]. In aging men, there is a steady reduction in the levels of testosterone [Citation5]. This has shown to lead to many clinical and major biological conditions that have been associated with low levels of testosterone (T) such as ischemic heart disease, hypertension, diabetes mellitus, hypercholesterolemia, and osteoporosis () [Citation6–9].
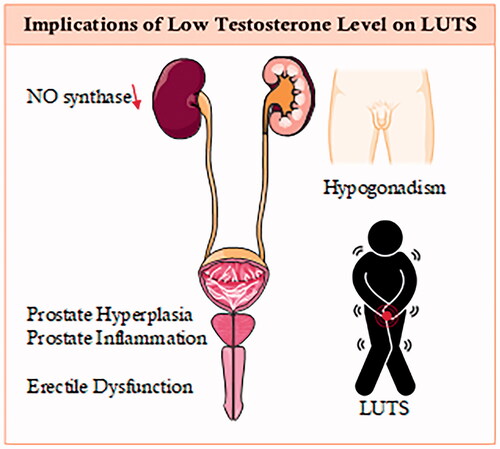
Figure 1. Implication of testosterone in men’s health.
Lower urinary tract symptoms (LUTS) are sequences of benign prostatic enlargement (BPE), or dynamic tension at bladder neck level leading to symptoms (obstructive, irritative, or both), The symptoms include intermittency, weak stream, hesitancy, urine frequency, nocturia, and urgency [Citation10,Citation11]. Many researchers have investigated testosterones’ role in male genital organs in terms of differentiation and development in prolonging good physical health [Citation12].
Even though several investigations were studied on the association between sex hormones and benign prostatic hyperplasia (BPH), few of them have reported the association between LUTS symptoms and circulating testosterone. Hypogonadism was observed in 20% of aging men with LUTS, but without any effect on the status of symptoms [Citation7,Citation13]. For instance, Litman et al. published a survey study on the possible association between testosterone and LUTS symptoms. Even though they reported good findings in terms of sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulphate (DHEAS), dihydrotestosterone (DHT), and oestradiol (E2), it was concluded that circulating levels of sex hormones are not significant predictors of urological symptoms and perhaps other factors control the pathophysiology of LUTS in hypogonadal men [Citation14]. Furthermore, looking at LUTS and serum sex steroid hormones, there seems to be no associations between LUTS symptoms with total and calculated free testosterone, however, there seem to be some links with androstanediol glucuronide, a dihydrotestosterone metabolite, and estradiol [Citation11].
Low T levels were found to be inversely correlated with urethral closure and detrusor pressure with maximum flow in patients with clinical bladder outlet blockage and also increased detrusor overactivity [Citation15].
Overall, it was proven to be difficult to link plasma testosterone (T) and LUTS. However, it is worth noting that in elderly males, both symptoms and signs of TD do not correlate consistently to testosterone concentrations [Citation16]. This could be explained (in part) by androgen receptor characteristics that were believed to be influenced by the number of CAG repetitions [Citation17].
The side effects of TD and the positive effects of testosterone therapy may be oblique. This review aims to show the latest updates of TTh in LUTS including potential adverse effects, advantages, and disadvantages. It shows the positive effect of regulating levels of testosterone in elderly men on metabolic syndrome (MetS) variables as well as the International Prostate Symptoms Score (IPSS) [Citation18] scores and with no scientific evidence that TTh could worse LUTS in men with hypogonadism.
Areas of concentration
Metabolic syndrome and LUTS
Even though the exact association and link between LUTS and MetS is still not completely clear, findings of fast developed LUTS symptoms or normally BPE surgery with men experiencing metabolic alterations support this possible hypothesis.
Pashootan et al. reported an observational cohort study on 4666 European men to investigate the association between MetS and LUTS and revealed a strong association in frequency and severity between LUTS and BPH and metabolic syndrome [Citation19]. Other reports attempted to further clarify some epidemiological relationships between LUTs and MetS and found that metabolic syndrome is linked to autonomic nervous system overactivity [Citation20,Citation21]. Although the vegetative nervous system and its overactivity are perhaps not to be the actual cause of LUTS, it majorly acts in progressing the level of severity in LUTS in addition to the intrinsic basal intensity, which is found to cause LUTS by the pathophysiological characteristics and genitourinary anatomical [Citation22]. In addition, Ullrich et al. published a study and found an association and evidence that stress is mainly associated with the progression of prostatic disease [Citation23]. Accordingly, higher BMI, age, in addition to a greater diastolic blood pressure reactivity were also found to be related to the postvoid residual bladder volume, total prostate gland volume, larger transition zone, in addition to more severity in LUTS [Citation23]. Inflammation penetrates are usually detected around and in the nodules for both benign and symptomatic BPH [Citation24]. Furthermore, metabolic syndrome could be the intermediary of this relationship and is possibly associated with a higher concentration of blood C-reactive protein, a non-specific measure of inflammation [Citation25]. Therefore, the relationship between the higher circulating C-reactive protein concentrations and metabolic syndrome to LUTS may be the direct indication of the symptomatic BPH intraprostatic inflammation [Citation24,Citation25]. Central obesity is also known as the key core of metabolic alterations, and therefore, increased abdominal adiposity contributes to hypertension, insulin resistance, dyslipidemia, type-II diabetes, and impaired glucose metabolism. Moreover, El-Sakka et al. found a solid link between low total testosterone levels and dehydroepiandrosterone sulfate (DHEA-S) in patients with badly controlled type-II diabetes [Citation26].
In addition, insulin resistance is mainly related to hyperinsulinemia, which contributed to the developing of cancer via a growth-promoting effect [Citation27,Citation28]. This could be a contributing factor in aging men’s prostate development [Citation27]. Clearly, normalizing T levels have been shown to modulate insulin sensitivity in hypogonadal men with or without noticeable changes in body composition [Citation29].
Although the elements of metabolic syndrome were found to be contributed to more development of LUTS and ED. ED risk factors and medical comorbidities were found to be common among LUTS patients [Citation30]. Consequently, it is not unexpected with the growing number of studies that have been published to find an association between ED and LUTS [Citation31,Citation32]. In addition, a possible underlying association of vascular with LUTS and ED can be also denoted [Citation33]. Other studies reported that a decrease in plasma testosterone levels, which is known as age-related, is found to be central to obesity and ED among aging men improved by restoring T to normal levels [Citation34–38]. Therefore, it is worth looking at the association of LUTS with late-onset hypogonadism (LOH), which is expected to appear, like the other disorders discussed, in the lives of senior men above 65 years at the same time.
Effects of testosterone on LUTS
Testosterone may not be the "primary mover" behind the effects of testosterone on urinary tract systems that are functionally and physically linked to LUTS. The indirect association could obscure the interrelationship between symptoms of LUTS and testosterone levels that is biologically feasible.
Androgen receptors were discovered in considerable quantities in the urethral and bladder epithelial cells [Citation39,Citation40]. Other researchers have assumed that suppressing detrusor activity is the impact of testosterone on postsynaptic non-genomic receptors [Citation41,Citation42].
Juan et al. reported a study on the role of castration (androgen deprivation) on lower urinary tract tissue enzymes of male rabbits and investigated the activities of citrate synthase-thapsigargin sensitive Ca2+ ATPase, and choline acetyltransferase as markers [Citation43]. They found that Ca2+ ATPase in the control corpora was higher than what was found in the control bladder or urethra. It was concluded that the differences significantly presented within the activities of Mitochondria, ATPase, and Choline acetyltransferase in the lower urinary tract associated organs. Studies that treated one illness (e.g. ED) in elderly men while also monitoring the role of others (e.g. LUTS) should agree on the consolidation of this link. However, a complete image of the influence of T on the lower urinary tract needs more investigation [Citation44].
The impact of testosterone on the bladder was first reported by Holmäng et al. [Citation45] and found that in the testosterone-treated group, the urinary flow was increased in the mean volume of urine compared to the placebo treated group. There has been initial evidence on LUTS and testosterone benefits for men according to positive results on data recorded on normalizing testosterone levels in hypogonadal males with BPH and LOH [Citation44]. Kalinchenko et al. found that testosterone treatment in men with SLOH positively affects bladder functions, which include decreasing detrusor pressure and increasing bladder capacity and compliance [Citation46]. A pilot study supports these findings as well, the study investigated the impact of testosterone undecanoate (TU) or TTh in hypogonadal men having LUTS with LOH [Citation47].
Even though other reports did not study the direct effect of testosterone therapy and LUTS and looked at testosterone therapy and the International Prostate Symptoms Score (IPSS) [Citation44], their findings showed some great advantages of TTh among the aging male. Accordingly, Saad et al. reported the effects of TU for more than 12 months and showed positive effects on IPSS and metabolic syndrome [Citation48]. Another study reported the testosterone gel (T gel) effectiveness for more than 9 months and revealed that higher plasma levels of testosterone that reduced the IPSS store were shown with T gel (50 mg/day) than with TU/TTh. This can possibly relate to the plasma levels of testosterone on LUTS [Citation49]. Furthermore, Okada et al. reported an improvement in LUTS among Japanese men with LOH after 6 months of taking TTh [Citation50].
All these studies showed that there were great benefits of using TU (50 mg/day) and testosterone gel in metabolic syndrome and in the IPSS. Additionally, there was also a better improvement in the IPSS for hypogonadal patients given TU/TTh after T gel treatment alone for 9 months period [Citation44].
Nitric oxide synthase in the urinary tract
Nitric oxide (NO), known as a non-cholinergic neurotransmitter, is reported to relax smooth muscles in both animals and humans is found in the genital tissues and the urinary tract [Citation44]. According to a human study by Ehrén et al., NO is reported as a micturition reflex and as an essential nerve-induced mediator of erection that works on the dilatation and erection of the bladder neck and urethral. Moreover, NO is also reported to be part of several other processes such as the human urogenital tract [Citation51].
NOS appears to be presented in up to 96% of neurons in the bladder wall in humans. The bladder body’s detrusor muscle has moderated innervation from nitric oxide synthase-immunoreactive nerve terminals, while muscles in the urethral were found to have a stronger innervation. NO could have also a major role in bladder neck relaxation as an inhibitory transmitter [Citation52].
Cyclic nucleotides are key nitric oxide secondary messengers that modulate the contraction of different muscles. Phosphodiesterases (PDEs) have an essential part in the regulating process of cyclic nucleotides, their action length and existence in the urine bladder in human was also reported [Citation53,Citation54].
For instance, Qiu et al. reported a study that looked at PDE5 activity and expression in the bladder. Authors found the role of PDE5 in modulating the bladder smooth muscle tone and accordingly, PDE5 is known as a nitric oxide/cGMP signaling inhibitor [Citation55]. Furthermore, Vardenafil appears to inhibit PDE5 activity, suggesting that it could be used to treat irritative LUTS [Citation55]. In addition, Filippi et al. reported that castration in the rat bladder is found to reduce PDE5 gene expression, but testosterone treatment restored it [Citation56].
NO-synthase proved to be androgen-dependent in the rat urogenital tract, adding to the evidence for androgens’ role in the urogenital tract [Citation57]. Meanwhile, several reports have conclusively shown that phosphodiesterase inhibitors help those patients with LUTS [Citation58,Citation59].
From the foregoing, androgens appeared not only important for the prenatal formation of the urogenital tract and puberty development, but also for maintaining the functionality of the urinary tract system in adulthood, like the penis erectile tissue. Therefore, the discomfort experienced by elderly men with micturition may be due to the decrease in testosterone levels as they age.
LUTS, androgens, and prostatic inflammation
Several immune cells exist in the prostate tissue that are immunocompetent such as granulocytes, lymphocytes, and macrophages, which upon activation can introduce a chronic immune response to persisting inflammations. T is known to help the main cytokines, for example, interleukin (IL)-2 and lymphocytes secrete interferon (IFN)-I, which are known to exist in the early phase of BPH [Citation60]. Furthermore, IL-17 and IFN-c, which are generated by macrophages and Lymphocytes, stimulate chemokine production using stromal cells, and therefore, induce BPH development and prostate cell proliferation [Citation61,Citation62]. The increased IL-6 and IL-8 secretion by prostate stromal cells, which are significant features in prostate hyperplasia, are known to be related to the increased levels of IL-17 [Citation63]. Several reports revealed that men having prostatitis are at a higher risk of LUTS, and therefore, this inflammation could be principal to BPH development [Citation64,Citation65]. For instance, Vignozzi et al. studied prostate inflammation by having low testosterone levels and high estrogen levels in male rabbits with metabolic syndrome (MetS) and concluded that hypogonadism has a major impact on prostate inflammation and its development [Citation66,Citation67]. In line with other reports, testosterone therapy could reduce inflammation and prevent further development of LUTS in men diagnosed with prostate inflammation and hypogonadism [Citation66,Citation68–70].
Temporary interruption of TTh
LUTS are known to be associated with hypogonadism in addition to other common conditions such as obesity, Ed, hypertension, CVD, and depression. Testosterone treatment therapy improves the hypogonadal patient’s metabolic factors and the withdrawal, or any interruption converses these benefits [Citation71,Citation72]. Clinical guidelines recommend men with hypogonadism be treated with TTh for a specific period such as 3 months/6 months/12 months and until benefits are achieved [Citation73]. Long-term treatment of testosterone therapy for 12 months may not be enough for achieving the maximum benefits of TTh [Citation72]. In addition, erectile function substantially improved for up to 9 years, although the stabilization of AMS after 2 years of treatment. It is recommended that patients, who were under TTh for up to 12 months and did not gain benefits continue for longer periods.
TTh in hypogonadal men improves residual bladder wall thickness, voiding volume, CRP, AMS, IPSS, and adiposity parameters, however, prostatic enlargement progressed with time, and the PSA also slowly raised accordingly [Citation70]. Interestingly, interruption of TTh replacement caused a deterioration of residual voiding volume, obesity parameters, and bladder wall thickness increased, AMS, and IPSS scores were also worse. It is clear that men are reliant on maintaining a physiological level of testosterone, and reducing the levels, leads to a deterioration of symptoms and worsening of the obesity state, with its consequences.
Improving LUTS independent of prostate size
Prostatic enlargement secondary to benign hyperplasia is one of the major causes of LUTs. The relationship between LUTs and testosterone is well-documented. Approximately 20% of men with hypogonadism experienced LUTs. If the LUTs severity is related to the levels of testosterone, then the restoration of T levels to the physiological levels using TTh would expect to change enzymes in the urinary tract tissue, and other pathology related to low levels of testosterone such as local ischemia and fibrosis. However, increased prostate volume and worsening urine function remain the main concern with TTh in elderly men and LUTS patients.
A study investigated the impact of TTh on LUTs with hypogonadism [Citation50]. Sixty hypogonadal men were involved in this study and were injected with 250 mg of T. No statistically significant alterations were observed after the TTh treatment in residual urine volume, BMI, and prostate volume. In addition, LUTs and particularly storage symptoms among the control group were significantly improved 6 months after TTh. It was concluded that the improved LUTs parameters appeared to be independent of prostate volume and others such as AMS and IIEF-s scores.
The safety of long-term TTh improves the voiding function, despite the prostatic size, and interruption worsens or reverts the improvement [Citation71,Citation74,Citation75].
Conclusion
Men tend to experience LUTS and other alignments like erectile dysfunction (ED) and metabolic syndrome very commonly and particularly in the aging male population. The association between LUTS and testosterone levels on an epidemiological level may be indirect. However, the autonomic nervous system overactivity may be related to the connection of LUTS to metabolic syndrome. Additionally, LUTS can be related to the genitourinary anatomical/pathophysiological characteristics, which is why it may become severe because of the autonomic nervous system overactivity. Long-term testosterone is found to be effective and safe for males’ metabolic outcomes. This review showed the positive effect of normalizing levels of testosterone in aging men on metabolic syndrome variables as well as the IPSS scores and with no scientific evidence that TTh could worsen LUTS in men with hypogonadism. TTh has been used worldwide as a therapy to treat testosterone deficiency with increasing evidence and benefits. Nevertheless, more randomized and controlled research and clinical trials are needed to further support the use of TTh in treating men with both severe LUTS and testosterone deficiency.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male. 2021;24(1):119–138.
- EAU Guidelines. Edn. Presented at: EAU Annual Congress Barcelona; 2019. EAU Guidelines Office, Arnhem, the Netherlands. ISBN 978-94-92671-04-2.
- Park J, Kim ES, Lee YJ, et al. Sex differences in lower urinary tract symptoms in older Korean adults living in rural areas: prevalence, quality of life, and associated factors. Int Neurourol J. 2018;22(3):212–219.
- Ko IG, Hwang L, Jin JJ, et al. Add-on therapy with the α-Blockers tamsulosin and naftopidil improves voiding function by enhancing neuronal activity in prostatic hyperplasia rats. Int Neurourol J. 2018;22(1):20–29.
- El-Sakka AI, Hassoba HM. Age related testosterone depletion in patients with erectile dysfunction. J Urol. 2006;176(6 Pt 1):2589–2593.
- Yassin DJ, El Douaihy Y, Yassin AA, et al. Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J Urol. 2014;32(4):1049–1054.
- Al-Zoubi RM, Yassin AA, Alwani M, et al. A systematic review on the latest developments in testosterone therapy: innovations, advances, and paradigm shifts. Arab J Urol. 2021;19(3):370–375.
- Alwani M, Al-Zoubi RM, Al-Qudimat A, et al. The impact of long-term testosterone therapy (TTh) in renal function (RF) among hypogonadal men: an observational cohort study. Ann Med Surg (Lond). 2021;69:102748.
- Al-Qudimat A, Al-Zoubi RM, Yassin AA, et al. Testosterone treatment improves liver function and reduces cardiovascular risk: a long-term prospective study. Arab J Urol. 2021;19(3):376–386.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876.
- Rohrmann S, Nelson WG, Rifai N, et al. Serum sex steroid hormones and lower urinary tract symptoms in third national health and nutrition examination survey (NHANES III). Urology. 2007;69(4):708–713.
- Lee MH, Shin YS, Kam SC. Correlation between testosterone replacement treatment and lower urinary tract symptoms. Int Neurourol J. 2021;25(1):12–22.
- Yassin DJ, Doros G, Hammerer PG, et al. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–1576.
- Litman HJ, Bhasin S, O’Leary MP, et al. An investigation of the relationship between sex-steroid levels and urological symptoms: results from the Boston area community health survey. BJU Int. 2007;100(2):321–326.
- Koritsiadis G, Stravodimos K, Mitropoulos D, et al. Androgens and bladder outlet obstruction: a correlation with pressure-flow variables in a preliminary study. BJU Int. 2008;101(12):1542–1546.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91(11):4335–4343.
- Zitzmann M. Mechanisms of disease: pharmacogenetics of testosterone therapy in hypogonadal men. Nat Clin Pract Urol. 2007;4(3):161–166.
- Salonia AB, Bettocchi C, Boeri L, et al. Reproductive, European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction, Eur Urol. 2021;80(3):333-357. DOI: 10.1016/j.eururo.2021.06.007
- Pashootan P, Ploussard G, Cocaul A, et al. Association between metabolic syndrome and severity of lower urinary tract symptoms (LUTS): an observational study in a 4666 european men cohort. BJU Int. 2015;116(1):124–130.
- Björntorp P, Rosmond R. The metabolic syndrome–a neuroendocrine disorder? Br J Nutr. 2000;83(Suppl 1):S49–S57.
- Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83(6):1853–1859.
- McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174(4 Part 1):1327–1333.
- Ullrich PM, Lutgendorf SK, Kreder KJ. Physiologic reactivity to a laboratory stress task among men with benign prostatic hyperplasia. Urology. 2007;70(3):487–491. Discussion 91–92.
- Rohrmann S, De Marzo AM, Smit E, et al. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the third national health and nutrition examination survey (NHANES III). Prostate. 2005;62(1):27–33.
- Teoh H, Verma S. C-reactive protein, metabolic syndrome, and end organ damage. Metabolism. 2007;56(12):1620–1622.
- El-Sakka AI, Sayed HM, Tayeb KA. Type 2 diabetes-associated androgen alteration in patients with erectile dysfunction. Int J Androl. 2008;31(6):602–608.
- Hammarsten J, Högstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39(2):151–158.
- Washino S, Hirai M, Saito K, et al. Impact of androgen deprivation therapy on volume reduction and lower urinary tract symptoms in patients with prostate cancer. Low Urin Tract Symptoms. 2018;10(1):57–63.
- Yialamas MA, Dwyer AA, Hanley E, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92(11):4254–4259.
- El-Sakka AI. Lower urinary tract symptoms in patients with erectile dysfunction: analysis of risk factors. J Sex Med. 2006;3(1):144–149.
- Rosen RC. Update on the relationship between sexual dysfunction and lower urinary tract symptoms/benign prostatic hyperplasia. Curr Opin Urol. 2006;16(1):11–19.
- Yassin A, Saad F, Hoesl CE, et al. Alpha-adrenoceptors are a common denominator in the pathophysiology of erectile function and BPH/LUTS–implications for clinical practice. Andrologia. 2006;38(1):1–12.
- Canguven O, Talib RA, El Ansari W, et al. Al-Ansari a testosterone therapy has positive effects on anthropometric measures, metabolic syndrome components (obesity, lipid profile, diabetes mellitus control), blood indices, liver enzymes, and prostate health indicators in elderly hypogonadal men. Andrologia. 2017;49(10):e12768.
- Allan CA, Strauss BJ, Burger HG, et al. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93(1):139–146.
- Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280–293.
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J Urol. 2006;176(4):1524–1528. Discussion 7–8.
- Kapoor D, Malkin CJ, Channer KS, et al. Androgens, insulin resistance and vascular disease in men. Clin Endocrinol (Oxf). 2005;63(3):239–250.
- Shabsigh R, Perelman MA, Lockhart DC, et al. Health issues of men: prevalence and correlates of erectile dysfunction. J Urol. 2005;174(2):662–667.
- Rosenzweig BA, Bolina PS, Birch L, et al. Location and concentration of estrogen, progesterone, and androgen receptors in the bladder and urethra of the rabbit. Neurourol Urodyn. 1995;14(1):87–96.
- Zitzmann M. Effects of testosterone replacement and its pharmacogenetics on physical performance and metabolism. Asian J Androl. 2008;10(3):364–372.
- Watkins TW, Keast JR. Androgen-sensitive preganglionic neurons innervate the male rat pelvic ganglion. Neuroscience. 1999;93(3):1147–1157.
- Hall R, Andrews PL, Hoyle CH. Effects of testosterone on neuromuscular transmission in rat isolated urinary bladder. Eur J Pharmacol. 2002;449(3):301–309.
- Juan YS, Onal B, Broadaway S, et al. Effect of castration on male rabbit lower urinary tract tissue enzymes. Mol Cell Biochem. 2007;301(1–2):227–233.
- Yassin AA, El-Sakka AI, Saad F, et al. Lower urinary-tract symptoms and testosterone in elderly men. World J Urol. 2008;26(4):359–364.
- Holmäng S, Mårin P, Lindstedt G, et al. Effect of long-term oral testosterone undecanoate treatment on prostate volume and serum prostate-specific antigen concentration in eugonadal Middle-aged men. Prostate. 1993;23(2):99–106.
- Kalinchenko S, Vishnevskiy EL, Koval AN, et al. Beneficial effects of testosterone administration on symptoms of the lower urinary tract in men with late-onset hypogonadism: a pilot study. Aging Male. 2008;11(2):57–61.
- Mskhalaya GT, Koval AN, Vishnevskiy EL, et al. The efficiency of testosterone undecanoat (Nebido) therapy on lower urinary tract symptoms (LUTS) in men with late-onset hypogonadism (LOH). Presented at: 1st European congress of the Society for the Study of the Aging Male; 2007.
- Saad F, Gooren L, Haider A, et al. An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch Androl. 2007;53(6):353–357.
- Saad F, Gooren LJ, Haider A, et al. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J Androl. 2008;29(1):102–105.
- Okada K, Miyake H, Ishida T, et al. Improved lower urinary tract symptoms associated with testosterone replacement therapy in Japanese men with late-onset hypogonadism. Am J Mens Health. 2018;12(5):1403–1408.
- Ehrén I, Adolfsson J, Wiklund NP. Nitric oxide synthase activity in the human urogenital tract. Urol Res. 1994;22(5):287–290.
- Smet PJ, Jonavicius J, Marshall VR, et al. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71(2):337–348.
- Qiu Y, Kraft P, Craig EC, et al. Identification and functional study of phosphodiesterases in rat urinary bladder. Urol Res. 2001;29(6):388–392.
- Werkström V, Svensson A, Andersson KE, et al. Phosphodiesterase 5 in the female pig and human urethra: morphological and functional aspects. BJU Int. 2006;98(2):414–423.
- Lenzi A, Balercia G, Bellastella A, et al. Epidemiology, diagnosis, and treatment of male hypogonadotropic hypogonadism. J Endocrinol Invest. 2009;32(11):934–938.
- Filippi S, Morelli A, Sandner P, et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology. 2007;148(3):1019–1029.
- Chamness SL, Ricker DD, Crone JK, et al. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995;63(5):1101–1107.
- Uckert S, Hedlund P, Andersson KE, et al. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future. Eur Urol. 2006;50(6):1194–1207.
- Sairam K, Kulinskaya E, McNicholas TA, et al. Sildenafil influences lower urinary tract symptoms. BJU Int. 2002;90(9):836–839.
- Steiner GE, Stix U, Handisurya A, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146.
- Macoska JA. Chemokines and BPH/LUTS. Differentiation. 2011;82(4–5):253–260.
- Lee JH, Kim Y, Park YW, et al. Relationship between benign prostatic hyperplasia/lower urinary tract symptoms and total serum testosterone level in healthy Middle-aged eugonadal men. J Sex Med. 2014;11(5):1309–1315.
- Steiner GE, Newman ME, Paikl D, et al. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56(3):171–182.
- Nickel JC. The overlapping lower urinary tract symptoms of benign prostatic hyperplasia and prostatitis. Curr Opin Urol. 2006;16(1):5–10.
- Hung SC, Lai SW, Tsai PY, et al. Synergistic interaction of benign prostatic hyperplasia and prostatitis on prostate cancer risk. Br J Cancer. 2013;108(9):1778–1783.
- Vignozzi L, Rastrelli G, Corona G, et al. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014;37(4):313–322.
- Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: where are we now? J Endocrinol Invest. 2011;34(3):232–243.
- Traish AM, Johansen V. Impact of testosterone deficiency and testosterone therapy on lower urinary tract symptoms in men with metabolic syndrome. World J Mens Health. 2018;36(3):199–222.
- Condorelli RA, Vicari E, Calogero AE, et al. Male accessory gland inflammation prevalence in type 2 diabetic patients with symptoms possibly reflecting autonomic neuropathy. Asian J Androl. 2014;16(5):761–766.
- Debruyne FMJ, Behre HM, Roehrborn CG, et al. Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: prostate health outcomes in the registry of hypogonadism in men. BJU Int. 2017;119(2):216–224.
- Yassin A, Nettleship JE, Talib RA, et al. Effects of testosterone replacement therapy withdrawal and re-treatment in hypogonadal elderly men upon obesity, voiding function and prostate safety parameters. Aging Male. 2016;19(1):64–69.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus - a series of case reports. Aging Male. 2015;18(3):164–168.
- Dohle GR, Arver S, Bettocchi C, et al. EAU Guidelines on Male Hypogonadism [cited 2015 Jun 25]. Available from: http://uroweb.org/guideline/malehypogonadism
- Saad F, Caliber M, Doros G, et al. Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality. Aging Male. 2020;23(1):81–92.
- Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf). 2016;84(1):107–114.
