Abstract
Some evidence have suggested that various nutrients and inflammatory factors might influence the lower urinary tract function. However, the correlation between diet and urinary flow rate (UFR) is not clear. Our study aimed to evaluate the association between the dietary inflammatory index (DII) and UFR. We performed a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES) database from 2009–2016. The dependent and independent variables were UFR and DII score, respectively. Dietary information was collected by 24-hour dietary recall interviews method, and DII scores were computed based on it. Tertiles group was divided according to DII scores. The study included 17,114 participants for whom data on DII and UFR were available, with a mean age of 35.68 ± 20.96 years. Participants with higher DII score presented lower UFR levels (β= −0.05; 95% CI: [−0.06–0.04]). In addition, the risk of UFR decline elevated significantly gradual across DII score tertiles (p for trend <0.001). Our findings revealed that increased intake of pro-inflammatory diet, as a higher DII score, is correlated with decreased UFR. These results might be useful for the public health system to provide primary prevention recommendations for lower urinary tract voiding problem, but further high-quality prospective research is needed.
Introduction
Urinary flow rate (UFR) represents the amount of urine discharged through the urethra per unit time in the state of natural urination, which is the rate of urination. UFR is a common noninvasive screening tool for evaluating and quantifying the lower urinary tract voiding process. It is mainly affected by detrusor contraction strength and outlet resistance of bladder [Citation1]. Therefore, uroflowmetry has an important clinical significance for the preliminary screening of lower urinary tract symptoms (LUTS).
Generally, most people’s diet patterns are characterized by pro-inflammatory or anti-inflammatory consumption. Dietary inflammation index (DII) is a standard scoring system developed by University of South Carolina researchers in the cancer prevention and control program, which is used to comprehensively measure the overall inflammatory effect of an individual’s diet [Citation2]. Many studies have reported that DII scores had a positive correlation with inflammatory biomarkers (e.g. interleukin-6 [IL-6], high-sensitivity C-reactive protein [hsCRP] and tumor necrosis factor [TNF]) [Citation3–5]. The DII has been confirmed to be associated with many systemic diseases, which include esophageal cancer, ovarian cancer, adverse psychological health, cardiovascular disease (CVD) and musculoskeletal disorders [Citation6–9].
Different dietary patterns may lead to changes of lower urinary tract function. The effect of inflammation on urodynamics has recently been confirmed. In many urinary system diseases, inflammation is the main driving force for symptoms, dysfunction, and bladder injury, such as bladder outlet obstruction (BOO), bladder dysfunction, and urinary tract infections [Citation10]. Furthermore, the research results of Jiang et al. showed that increased suburothelial inflammation is associated with detrusor underactivity (DU) [Citation11], which may be one of the reason of UFR decline. In addition, relevant studies have also shown that inflammation may be the cause of BOO, and can further exacerbate the development of bladder dysfunction [Citation12].
As far as we know, there is no study to explore the relationship between inflammatory diet patterns and UFR. Therefore, our study aimed to evaluate the effect of DII on UFR using data from the NHANES. We hypothesized that increasing inflammatory potential of diet which represented by higher DII scores is associated with the decline of UFR.
Materials and methods
Data collection and participants selection
A regular and population-based cross-sectional research was performed, which used data from the NHANES. By designing a complex, stratified, multistage and probabilistic cluster sampling, the dataset provides estimates of the prevalence of a range of common diseases, which have been proven to be suitable for representative statistics. For the present study, we conducted four survey cycles (i.e. 2009–2010, 2011–2012, 2013–2014 and 2015–2016), and considered the weight in the analysis of the above data, which can improve the accuracy of the research and reduce the sampling survey error.
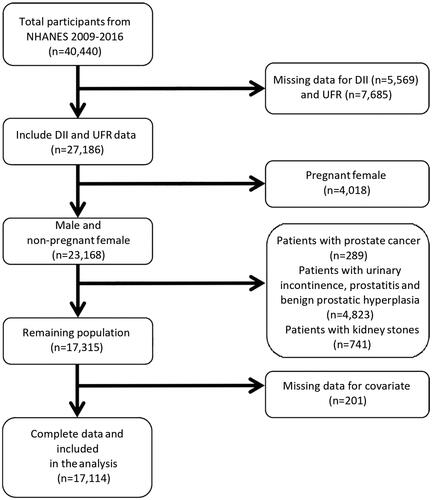
The total number of participants in these four 2-year cycles was 40,440. We screen participants according to the inclusion and exclusion criteria (). Eventually, 17,114 eligible individuals of the NHANES survey were included in our study.
Figure 1. Flowchart of participant inclusion and exclusion for analysis.
After explaining the whole research process, the whole informed consents of each qualified participant were obtained. All experimental methods were carried out according to the relevant guidelines and regulations of the Center for Disease Control and Prevention (CDC).
Exposure and outcome definitions
We assessed the dietary intake information by using the food intake survey data obtained through an individual 24-h dietary recall interviews method used in the NHANES. This dietary data was digitally processed by using the USD’s Food and Nutrient Database for Dietary Studies. The inflammatory potential of the diet was evaluated using the DII®, an index developed by Shivappa et al. to quantify the effect of diet on inflammation [Citation2]. In this study, 27 of the 45 food parameters were available through NHANES data, including alcohol, protein, fiber, fat, carbohydrate, cholesterol, omega3 and omega6 polyunsaturated fatty acids (PUFA), saturated fatty acids/monounsaturated fats (MUFA)/PUFA, magnesium, niacin, zinc, iron, riboflavin, folic acid, β-carotene, caffeine, selenium, thiamine and vitamins A, B6, B12, C, D and E. Several previous studies have validated the use of the 27 parameters for the DII score, and their inflammatory effect scores are shown in [Citation13–15]. Finally, all scores were summed from all food parameters to calculate the overall DII score. The process of DII score calculation was described in our previous research [Citation16]. For the assessment, positive scores indicate more pro-inflammatory diets, while more negative values are more anti-inflammatory [Citation2].
The UFR was measured by uroflowmetry (mL/min), and the data of three measurements are usually collected. The calculation formula of UFR is UFR = V/t, where t is the time elapsed between two urine voids and V is the volume of the second void [Citation17]. The participants had to record their last urination time before coming to the mobile examination center (MEC). Then, they would record the voiding time and volume of the urine sample and calculate the UFR for three times. The specimens were collected in different containers to guaranteeing enough data for various analyses. The composite UFR (mL/min) was measured by dividing the total urine volume collected by the total time covered by all collected voids [Citation18].
Covariates
Potential covariates were predefined based on the literature review [Citation19,Citation20]. The following confounders were summarized in multivariable adjusted models: continuous variables consisted of age, poverty to income ratio (PIR), body mass index (BMI), energy, categorical variables included gender (male/female), marital (married or living with partner/single), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican-American and others), insurance, smoking, alcohol intake per week, physical activity. BMI was calculated based on self-reported weight and height measurements and was classified as <25, 25–30, or >30 kg/mCitation2. CAD was also a potential confounder as it could affect both DII and UFR. To avoid multicollinearity, we generated a comprehensive CAD variable that aggregated multiple risk factors. One score was assigned to each of the three current risk factors: history of hypertension, coronary heart disease, or stroke. Additionally, two points were added for diabetes. Scores ranged from 0 to 5 [Citation21]. Meanwhile, all people were obtained the urine sample according to the standard procedure. In order to avoid errors caused by urine concentration, we have standardized creatinine for albumin in urine. What is more, considering the offset caused by the inclusion of different year-cycle, we also analyzed the year-cycle as a covariate. So we could acquire other confounders (i.e. albumin, total cholesterol level and serum creatinine) through some standard methods. More information can be found in the CDC laboratory procedure manual.
Statistical analysis
The statistical analysis was performed according to the CDC analytical reporting guidelines for complex NHANES data analysis (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). A sample weight was assigned to each person participating in NHANES. Therefore, we accounted for masked variance and used the proposed recommended weighting methodology. Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed in frequency or as a percentage. We regarded the DII score as a continuous variable and analyzed it using the weighted linear regression model, and then divided it into three groups (T1, T2 and T3) from the total sample size using the weighted chi-square test to calculate the difference between different DII groups (tertiles).
To investigate whether DII is correlated with UFR in selected participants, our statistical analyses consisted of three main steps. First, weighted univariate and multivariate linear regression models were employed. We estimated three models: crude model, no covariates were adjusted; model I, only adjusted for gender, age and race data; in the final model (model II), model I + other covariates presented in (i.e. BMI; PIR; insurance; marital; alcohol intake per week; physical activity; energy (kcal) and smoking). Moreover, to address for the non-linear association of DII and UFR, a weighted generalized additive model (GAM) and smooth curve fitting (penalized spline method) were performed. The subgroup analyses were then performed using weighted stratified logistic regression models to further determine the correlation between DII and UFR. In addition, in order to further explore the impact of various covariates on the association between DII and UFR, an interaction term was added to test the heterogeneity of association between the subgroups of interest.
Table 1. Baseline characteristics of participants.
All analyses were performed using the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empower stats.com, X&Y Solutions, Inc., Boston, MA). All P values less than 0.05 (two-sided) were considered statistically significant.
Results
Baseline characteristics of participants
A total of 17,114 participants were included in this study, with a weighted mean age of 35.68 ± 20.96 (20–65) years and males representing 68.83%. DII scores ranged from −5.18 to+5.54. The weighted distribution of selective participants sociodemographic characteristics and other covariates for the selected NHANES participants from 2009 to 2016, according to DII score tertiles (See ). At the same time, the changes in energy intake of the population in each cycle can be observed in Supplementary Figure 1.
Multivariate linear regression analysis
Our multivariate linear regression analysis noted that DII score negatively correlated with UFR (β= −0.05; 95% CI: [−0.06–−0.04]). Compared to participants in T1 of DII, there was a significantly increased risk of reduction of UFR for participants in T2 and T3 group who got higher DII score in the non-adjusted model (model I, β= −0.16; 95% CI: [−0.22, −0.10]; β= −0.28; 95% CI: [−0.33, −0.22]), minimally adjusted model (model II, β= −0.11; 95% CI: [−0.17, −0.05]; β= −0.19; 95% CI: [−0.26, −0.12]) and fully adjusted model (model III, β= −0.11; 95% CI: [−0.17, −0.05]; β= −0.19; 95% CI: [−0.26, −0.12]). Furthermore, the risk of reduction of UFR elevated significantly gradual across DII score tertiles (p for trend < 0.001) (See ).
Table 2. Association of dietary inflammatory index with urinary flow rate.
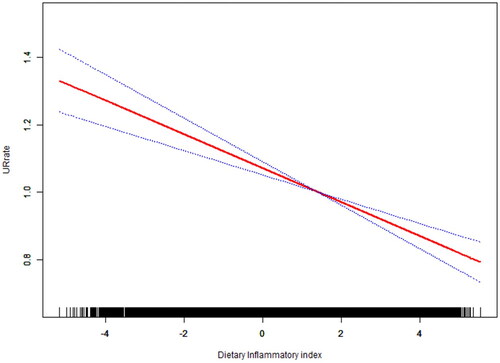
Non-linearity analysis and subgroup analyses
It is essential to analyse non-linear relationships for continuous variables. Thus, we also analyzed the non-linear relationship between DII and UFR and its recurrence. The result was negative indicating that there was no nonlinear relationship between the exposure variable and outcome in our research (). Then, we tested interactions with all covariates presented in . We did not observe significant differences in population analysis between subgroups with different demographic characteristics (race, age, gender). In addition, we found a significant relationship between DII and UFR among subgroups with BMI. Similarly, we conducted subgroup analysis of covariates in the lifestyle category, and no statistical significance was found among the interaction tests for characteristics associated with DII (P for interaction > 0.05). Meanwhile, we further conducted a sensitivity analysis on the included year-cycle, and the results showed that the correlation between DII and UFR is still significant, indicating that the negative correlation between DII and UFR was stable and reliable in our study (Supplementary Table 2).
Table 3. Stratified logistic regression analysis to identify variables that modify the correlation between DII and urinary flow rate.
Discussion
This cross-sectional study investigated the interaction between the food-based dietary index (DII score) and UFR to clarify the relationship between dietary potential inflammation and urination function using data from the NHANES. The main finding and novelty of our study is that the consumption of more pro-inflammation diets was negatively associated with attenuating of UFR after adjusting for a variety of potential confounders in both men and women.
Some related studies have been previously reported, and our research adds new evidence to this field. Liao et al. [Citation22] discussed the relationship between DII and urination function based on the NHANES database. In contrast, our study has increased the number of cycles included in the study year and has a larger sample size. At the same time, LUTS are mainly reflected by subjective feelings, while UFR serves as an objective indicator of lower urinary tract urination function, which can more intuitively reflect the damage of DII to urination function, providing good predictive indicators and data support for the improvement of clinical dietary structure in the future. In addition, Li et al. [Citation23] studied the relationship between prostatitis and clinical parameters related to prostate hyperplasia by collecting samples from patients with or without prostate hyperplasia. In contrast, our study is a cross-sectional study that includes a large population, representing the national population, and explores the correlation between DII and UFR. At the same time, DII may cause systemic inflammatory status. It explores the possible causes of inflammation affecting urination function from multiple perspectives, such as the impact on bladder function, rather than just prostate hyperplasia. Moreover, our study proposes the impact of inflammation on lower urinary tract voiding dysfunction from another perspective, providing a new idea for the clinical treatment of lower urinary tract voiding dysfunction in the future. Similarly, Chiu et al. [Citation24] also explored the relationship between UFR and muscle strength based on the NHANES database, and this study explained the possible reasons for the decrease in UFR from the perspective of bladder contractility. Our study elucidates the possible reasons for the decrease in UFR caused by high DII from the perspectives of bladder outlet obstruction and bladder dysfunction, providing insights into the possible mechanisms by which DII affects UFR in the future. Additionally, Ko et al. [Citation25] explored the effect and safety of sildenafil with different doses and drug modes on improving LUTS caused by benign prostatic hyperplasia (BPH). Our research objective is to explore the correlation between DII and UFR based on a nationally representative population, provide possible mechanisms of lower urinary tract dysfunction from an inflammatory perspective, and provide new dietary approaches for the clinical treatment of lower urinary tract dysfunction. Besides, the study conducted by Cakir et al. [Citation26] suggested that statins are recommended for patients with BPH and metabolic syndrome. In our subgroup analysis, we observed that individuals with a BMI > 30 had no impact on the main outcome, which may provide data support for future comprehensive treatment of BPH. Meanwhile, the relationship between DII and UFR shown in our study can also provide a new perspective for the comprehensive treatment of BPH.
The mechanism of the urination reflex is complex, which is affected by nerve conduction, detrusor function, and the bladder outlet. In neuromodulation, related studies have found that when the expression of inflammatory factors in cell micro-environment is up-regulated, it can activate nuclear factorκB (NF-κB) pathway and induce cell apoptosis; on the other hand, inflammatory factors can stimulate nerve cells to start self-protection and repair mechanism, among which TNF -αand IL-6 can cause degeneration and demyelination of peripheral nerve axis, causing nerve conduction dysfunction [Citation27,Citation28]. In terms of muscle control, detrusor and pelvic floor muscles, which are the main muscles affecting urination. It has been reported that IL-1 can inhibit glucose transport and lactic acid production in muscle tissue, resulting in muscle metabolic disorders and inhibition of myoblast proliferation, fusion, and regeneration, finally leading to myasthenia [Citation29]. Meanwhile, a recent study found that the weak urination strongly associated with the presence of lower muscle strength, which including both smooth muscle and skeletal muscle [Citation24].
Some previous studies have shown that dietary patterns and urination functions are closely related. For example, a recent research suggested that a decreased risk of voiding dysfunction was found in relation to fish and olive oil, which contain lots of n-3 polyunsaturated fatty acids (n-3 PUFA) [Citation30]. Most of the metabolites of n-3 PUFA have anti-inflammatory effect. Among them, Resolvin-E1 (RvE1) can inhibit the activation of neutrophils, prevent the migration of neutrophils across the endothelium, induce apoptosis of neutrophils, promote the non-inflammatory clearance of neutrophils by macrophages in inflammatory sites, and reduce the production of IL-12 [Citation31,Citation32]. Moreover, Das et al. [Citation33] explored that arachidonic acid is the major precursor of inflammation. Therefore, foods with high saturated fat and cholesterol which rich in arachidonic acid have higher DII score. It is benefit for voiding dysfunction patients to reduce the intake of these foods whose metabolites are inflammatory triggers. Furthermore, in the NHANS III survey, 2,337 men aged 60 years and older had a borderline correlation between lower urinary tract voiding symptoms and serum CRP, which is a marker of inflammation [Citation34].
However, some of the current findings are unconvincing. For example, it seems that vegetables and fruits, as well as anti-inflammatory micronutrients and anti-oxidants such as vitamins A and C and carotenoids are associated with lower urinary tract urination risk, which has not been confirmed by clinical trials. Since numerous factors which have anti-inflammatory properties are associated with the lower dysuria risk, these observations are consistent with a increasing assumption that inflammation has a pathological effect on lower urinary tract voiding [Citation35]. Thus, it is necessary to understand the effect inflammation on the UFR decline, although no definite conclusion can be drawn today, which is a mature research field especially as the potential dietary inflammation can be calculated by DII®. In addition, there is an urgent need for clinical trials to examine dietary interventions to regulate lower urinary tract voiding symptoms or prevent deterioration.
To our knowledge, this is the first study revealing the relationship between food-based DII score and UFR prevalence in a nationally representative sample. It should be noted that, it was a cross-section study, not longitudinal. Therefore, we are unable to account for dietary changes, which may change over time and the temporality of DII and UFR was unclear. In addition, the results that we found in this study between DII score and UFR may not be extended to people with very different dietary patterns. It is also worth noting that the results of UFR are influenced by psychological and environmental factors [Citation2]. What is more, the dietary intake is self-reported with recall bias and is estimated based on 24-h history, which limits the ability to accurately describe individuals’ habitual diets. Then, although we have adjusted for several potential confounders, we can not completely exclude the residual confounding by unmeasured or unknown variables. Above associations have been biologically proven, however, they need to be interpreted with caution, and confirmatory longitudinal studies or clinical trials are needed.
Conclusion
Our findings revealed that increased intake of pro-inflammatory diet, as a higher DII score, is correlated with decreased UFR. These results might be useful for the public health system to provide primary prevention recommendations for lower urinary tract voiding problem, but further research is needed.
Informed consent
Yes.
Author contributions
Yifan Li contributed to the design of the study, was responsible for data processing and analysis, and drafted the initial manuscript. Xianghong Zhou, Shi Qiu, Kun Jin, Xingyu Xiong and Sheng Wang decided the methods of data analysis. Zhou is also responsible for determining whether the data should be included in the discharge criteria. Qiang Wei and Lu Yang conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript submitted. All the authors read and approved the final manuscript.
Supplemental Material
Download JPEG Image (555.7 KB)Supplemental Material
Download MS Word (14.4 KB)Supplemental Material
Download MS Word (15.2 KB)Supplemental Material
Download MS Word (12.2 KB)Acknowledgments
The authors appreciate the American Centers for Disease Control and Prevention for conducting the survey and for providing the data available online freely, and all the participants for providing these data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data described in the manuscript, code book, and analytic code will be publicly and freely available without restriction at http://www.cdc.gov/nchs/nhanes.htm
Additional information
Funding
References
- Parekh DJ, Pope JC, 4th, Adams MC, et al. The use of radiography, urodynamic studies and cystoscopy in the evaluation of voiding dysfunction. J Urol. 2001;165(1):215–218.
- Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696.
- Na W, Kim M, Sohn C. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in korean: data from the health examinee cohort. J Clin Biochem Nutr. 2018;62(1):83–88.
- Shivappa N, Hébert JR, Rietzschel ER, et al. Associations between dietary inflammatory index and inflammatory markers in the asklepios study. Br J Nutr. 2015;113(4):665–671.
- Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25(6):398–405.
- Shivappa N, Hébert JR, Zucchetto A, et al. Dietary inflammatory index and endometrial cancer risk in an italian case-control study. Br J Nutr. 2016;115(1):138–146.
- Ramallal R, Toledo E, Martínez-González MA, et al. Dietary inflammatory index and incidence of cardiovascular disease in the SUN cohort. PLoS One. 2015;10(9):e0135221.
- Phillips CM, Shivappa N, Hébert JR, et al. Dietary inflammatory index and mental health: a cross-sectional analysis of the relationship with depressive symptoms, anxiety and well-being in adults. Clin Nutr. 2018;37(5):1485–1491.
- Cervo MM, Shivappa N, Hebert JR, et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr. 2020;39(2):516–523.
- Hughes FM, Jr, Harper SN, Nosé BD, et al. Specialized proresolution mediators in the bladder: annexin-A1 normalizes inflammation and bladder dysfunction during bladder outlet obstruction. Am J Physiol Renal Physiol. 2021;321(4):F443–F454.
- Jiang YH, Kuo HC. Urothelial barrier deficits, suburothelial inflammation and altered sensory protein expression in detrusor underactivity. J Urol. 2017;197(1):197–203.
- Jiang YH, Jhang JF, Hsu YH, et al. Potential urine biomarkers in bladder outlet obstruction-related detrusor underactivity. Tzu Chi Med J. 2022;34(4):388–393.
- Kim HS, Kwon M, Lee HY, et al. Higher Pro-Inflammatory dietary score is associated with higher hyperuricemia risk: results from the Case-Controlled korean genome and epidemiology study_cardiovascular disease association study. Nutrients. 2019;11(8):1803.
- Lee S, Quiambao AL, Lee J, et al. Dietary inflammatory index and risk of breast cancer based on hormone receptor status: a Case-Control study in korea. Nutrients. 2019;11(8):1949.
- Shivappa N, Bosetti C, Zucchetto A, et al. Association between dietary inflammatory index and prostate cancer among italian men. Br J Nutr. 2015;113(2):278–283.
- Zhang C, Bian H, Chen Z, et al. The association between dietary inflammatory index and sex hormones among men in the United States. J Urol. 2021;206(1):97–103.
- Middleton DR, Watts MJ, Lark RM, et al. Assessing urinary flow rate, creatinine, osmolality and other hydration adjustment methods for urinary biomonitoring using NHANES arsenic, iodine, lead and cadmium data. Environ Health. 2016;15(1):68.
- Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 2015;123(4):293–300.
- Ahluwalia N, Andreeva VA, Kesse-Guyot E, et al. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39(2):99–110.
- Ruiz-Canela M, Zazpe I, Shivappa N, et al. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvención con DIeta MEDiterránea) trial. Br J Nutr. 2015;113(6):984–995.
- Golub JS, Brickman AM, Ciarleglio AJ, et al. Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol Head Neck Surg. 2020;146(1):57–67.
- Liao X, Bian H, Zheng X, et al. Association of the inflammatory potential of diet and lower urinary tract symptoms among men in the United States. Aging Male. 2021;24(1):72–79.
- Li J, Li Y, Cao D, et al. The association between histological prostatitis and benign prostatic hyperplasia: a single-center retrospective study. Aging Male. 2022;25(1):88–93.
- Chiu HT, Kao TW, Peng TC, et al. Average urinary flow rate and its association with handgrip strength. Aging Male. 2020;23(5):1220–1226.
- Ko WJ, Han HH, Ham WS, et al. Daily use of sildenafil 50mg at night effectively ameliorates nocturia in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: an exploratory multicenter, double-blind, randomized, placebo-controlled study. Aging Male. 2017;20(2):81–88.
- Cakir SS, Ozcan L, Polat EC, et al. Statins are effective in the treatment of benign prostatic hyperplasia with metabolic syndrome. Aging Male. 2020;23(5):538–543.
- Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473.
- Fromont A, De Seze J, Fleury MC, et al. Inflammatory demyelinating events following treatment with anti-tumor necrosis factor. Cytokine. 2009;45(2):55–57.
- Dalakas MC. The molecular pathophysiology in inflammatory myopathies. Rev Med Interne. 2004;25(Suppl 1):S14–S6.
- Grammatikopoulou MG, Gkiouras K, Papageorgiou SΤ, et al. Dietary factors and supplements influencing prostate Specific-Antigen (PSA) concentrations in men with prostate cancer and increased cancer risk: an evidence analysis review based on randomized controlled trials. Nutrients. 2020;12(10):2985.
- Schwab JM, Chiang N, Arita M, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874.
- Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179(10):7021–7029.
- Das K, Buchholz N. Benign prostate hyperplasia and nutrition. Clin Nutr ESPEN. 2019;33:5–11.
- Rohrmann S, De Marzo AM, Smit E, et al. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the third national health and nutrition examination survey (NHANES III). Prostate. 2005;62(1):27–33.
- Gacci M, Vignozzi L, Sebastianelli A, et al. Metabolic syndrome and lower urinary tract symptoms: the role of inflammation. Prostate Cancer Prostatic Dis. 2013;16(1):101–106.