Abstract
Background
Primary urothelial carcinoma in the prostate (UCP) is extremely rare and occurs most frequently in the bladder. There are only dozens of primary cases reported in the literature. Here, we describe a rare case of primary UCP and review the literature.
Case presentation
A 67-year-old widowed male, was referred to our hospital due to the frequency, and urgency of dysuria. Magnetic resonance imaging (MRI) examination revealed prostate size was about 57 mm × 50 mm × 54 mm, increased prostatic transitional zone, and surrounding of prostatic duct indicate bar isointense T1, short T2, hyperintense DWI, and hyposignal ADC (PI-RADS 3); posterior of peripheral zone indicate patchy isointense T1, short T2, hyperintense DWI, and hyposignal ADC (PI-RADS 5). Subsequently, the patient underwent a transrectal prostate biopsy. Histopathological and immunohistochemical (IHC) assessments showed prostatic high-grade urothelial carcinoma with benign prostatic hyperplasia. Finally, the patient underwent laparoscopic radical prostatectomy. Four months after surgery, CT plain and enhanced scan revealed thickening of the bladder wall. On further workup, cystoscopy revealed lymphoid follicular changes in the cut edge of the radical prostatectomy, and cystoscopic biopsies showed the malignant tumor.
Conclusions
Prostatic urothelial carcinoma should always be considered if the patient with severe lower urinary tract symptoms or hematuria, PSA, and digital rectal examination without abnormalities, without a personal history of urothelial cancer, but contrast-enhanced MRI showed the lesion located in the prostate. As of right now, radical surgical resections remain the most effective treatment. The effectiveness of neoadjuvant or adjuvant chemotherapy is still controversial.
1. Introduction
Known as urothelial carcinoma, it can occur either in the upper urinary tract (renal pelvis and ureter) or in the lower urinary tract (bladder and urethra). However, primary urothelial carcinoma in the prostate (UCP) is extremely rare. The incidence of UCP has increased with the aging of the population and the development of better diagnostic techniques. Nevertheless, it accounts for only 1–4% of all prostate malignancies [Citation1]. In the literature, there are currently dozens of primary cases reported, with the first being reported by Ende et al. [Citation2]. Previous study has demonstrated that primary UCP is more aggressive compared to prostate adenocarcinoma [Citation3]. The clinical features of the disease mainly manifest as hematuria, dysuria, and normal PSA serum levels mostly, predominantly affecting the elderly. Besides, UCP can easily penetrate into bladder-neck tissue, even the bladder surrounding soft tissue due to excellent tissue penetration capability. It may pose UCP diagnostic difficulties and sometimes lead to delays because of the above-mentioned reasons. Approximately half of these UCP patients were in Stage T3 or T4, and 20% with distant metastatic disease. Common metastatic sites include the bones, lungs, and liver. Compared with prostate adenocarcinoma, but, bone metastases due to UCP are predominantly osteolytic.
Definitive diagnosis is reliant on histopathological and immunohistochemical (IHC). However, its’ serum PSA level was negative, which led to many patients not providing further nuclear magnetism imaging for prostate and digital rectal examination. Finally, the diagnostics are delayed as a result, which could have critical consequences.
Thus, scientists struggled to determine its epidemiology, etiology, and factors affecting prognosis, as well as an optimal therapeutic intervention. Currently, there is no consensus regarding the best curative option (radical cystoprostatectomy or radical prostatectomy) for primary UCP. Whether neoadjuvant chemotherapy or postoperative adjuvant chemoradiotherapy can improve the patients’ prognosis remains controversial.
Hence, we describe a case of a patient, with severe lower urinary tract symptoms, but thankfully positive imaging findings. So, he underwent transperineal prostate biopsy under general anesthesia, and was confirmed as primary high-grade UCP. Finally, he also underwent laparoscopic radical prostatectomy under general anaesthesia. In this article, we will present a case study and literature review to assist with the diagnosis and treatment of this tumor.
2. Case report
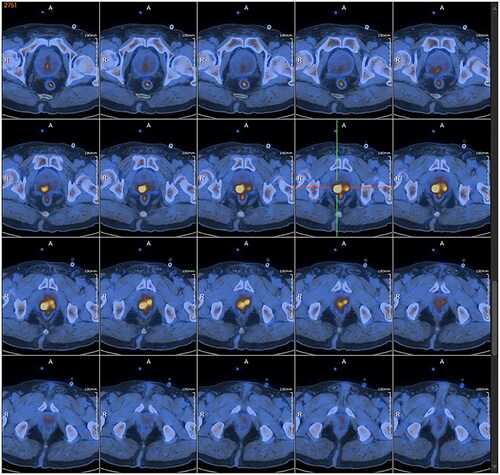
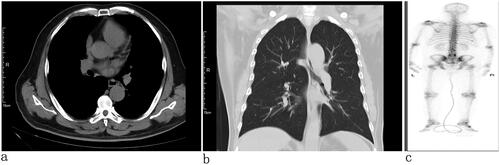
The patient, a 67-year-old widowed male, was referred to our hospital on 17 January 2023 due to the frequency, and urgency with dysuria about 6 months. The micturition problems were feelings of frequency, urgency, increased nocturia (6–7/night), gracile urine stream, and intermittent gross hematuria. The patient also had no past tumor history, and he also denied smoking and drinking in the past. We could find a prostate enlargement by digital rectal examinations. He presented nonpalpable nodules of the prostate. Urine Routine: white cell (314.30/µL↑; normal range, 0–30/µL), red blood cell (1803.70/L↑; normal range, 0–25/µL), urine leukocytes (3+), urine occult blood (3+). Lactate dehydrogenase (LDH 162.40 U/L; normal range, 120–250 U/L), total-prostate specific antigen (tPSA 2.76 ng/mL; normal range, 0–4 ng/mL), free prostate-specific antigen (fPSA 0.45 ng/mL; normal range, 0–0.934 ng/mL), tPSA/fPSA (f/tPSA 0.16). Magnetic resonance imaging (MRI) examination revealed prostate size was about 57 mm × 50 mm × 54 mm, increased prostatic transitional zone, and surrounding of prostatic duct indicate bar isointense T1, short T2, hyperintense DWI, and hyposignal ADC (PI-RADS 3); posterior of peripheral zone indicate patchy isointense T1, short T2, hyperintense DWI, and hyposignal ADC (PI-RADS 5) (). The thoracic CT and whole-body bone scintigraphy revealed no metastasis ().
Figure 1. MRI: prostate size was about 57 mm × 50 mm × 54 mm, increased prostatic transitional zone, and surrounding of prostatic duct indicate bar isointense T1 and short T2; posterior of peripheral zone indicate patchy isointense T1 and short T2.
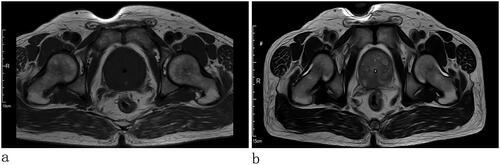
Figure 2. Chest CT: no tumor in the lungs and pleura (a) transverse view and (b) coronal view). (c) Whole body bone scan: no bone metastasis.
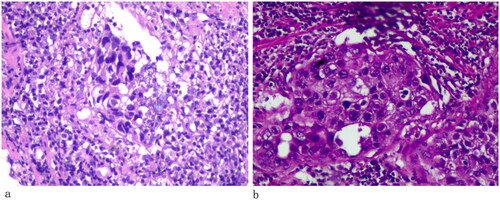
Subsequently, the patient underwent transrectal prostate biopsy under general anesthetic on 8 February 2023 due to lower limb venous thrombosis. A further image was done with positron emission tomography-CT (PET/CT) revealed the prostate protruding into the bladder was enlarged, with abnormal increases in glucose metabolism of the local lesion, considered to be a malignant tumor, and hypermetabolic pelvic lymph nodes, which should be considered for metastasis (). The pathologic report showed a prostatic high-grade urothelial carcinoma with benign prostatic hyperplasia (). Immunohistochemical: PSA (−), P63 (+), 34βE12 (+), GATA-3 (+), SATB2 (−), CK7 (+), CK20 (+), Ki-67 (5%), and P53 (strong+). The patient underwent laparoscopic radical prostatectomy under general anesthesia on 17 February 2023. Pathological examination revealed high-grade urothelial carcinoma (). The tumor invaded the prostatic parenchyma, some tumor tissue immediately next to the electrocautery margin, bilateral vas deferens was negative, and bilateral vas deferens and seminal vesicles did not show any tumor infiltrates. Tumors also affect the prostatic urethra. Immunohistochemical: PSA (−), P63 (+), 34βE12 (+), GATA-3 (+), SATB2 (−), CK7 (+), CK20 (+), Ki-67 (5%), and P53 (strong+). The postoperative pathological diagnosis was primary prostate high-grade urothelial carcinoma with benign prostatic hyperplasia. Postoperatively the patient was discharged on the 4th post-operative day. Four months after surgery, CT plain and enhanced scan revealed thickening of the bladder wall. On further workup, cystoscopy revealed lymphoid follicular changes in the cut edge of the radical prostatectomy, and cystoscopic biopsies showed a malignant tumor. Currently, the patient is on follow-up.
3. Discussion
3.1. Etiology and clinical presentation
Primary UCP arises from the prostatic urethral and prostatic duct, which is an extremely rare prostate malignant neoplasm and the literature is extremely sparse. Therefore, we report a case of a patient, with severe lower urinary tract symptoms, but thankfully positive imaging findings. Then, he underwent a transperineal prostate biopsy under general anesthesia, and was confirmed as primary high-grade UCP. Finally, he also underwent laparoscopic radical prostatectomy under general anesthesia. To our knowledge, UCP is mainly a secondary bladder tumor and is relatively common in advanced bladder disease. The incidence rate of primary UCP is only 1–4% [Citation1].
Its pathogenesis is still unclear due to its rarity. In the prostate and periurethral glands, columnar epithelium gradually changes to transitional cell epithelium. Some authors have suggested that primary transitional cell carcinoma of the prostate originates here [Citation4]. Karpas and Moumgis [Citation5], however, concluded that they are derived from reserve cells found between the basement membrane and luminal epithelium. Carcinoma cells are confined to prostatic intraductal in early phases, which leads to PSA being normal, even MRI and cystourethroscopy make it difficult to grasp the lesion. Some patients do not have any symptoms, and prostate tissue is floccose when prostate transurethral resection. With the development of the disease, patients will have symptoms, such as dysuria, urinary frequency, urgency, nocturia, and hematuria. Digital rectal examination lacks a remarkable typical presentation of early prostate cancer, and the prostate is hard and nodules are palpable in the advanced stage. MRI could show a space-occupying lesion at the prostate base and transitional region. UCP will be confirmed by prostate transurethral resection and prostate biopsy [Citation6].
3.1.1. Imaging examination
The imaging investigation of choice for primary UCP is transrectal ultrasonography, which can reveal prostate size, nodules, and structures of the prostate and the surrounding tissues. CT and MRI can further observe tumor infiltration, regional lymph node metastasis, and distant metastatic spread, which is of great importance to tumor stage. MRI, especially, has a high soft-tissue resolution and 3D imaging features, and it has a wide variety of imaging sequences without radiation damage. Tumor, located in the glandular tube, however, is difficult to detect. Therefore, it is difficult to be discovered and precisely diagnosed. Compared with X-ray, a bone scan can detect bone metastasis about 3–6 months in advance. Cystourethroscopy evaluates not only prostate size, space-occupying lesion, and local tumor condition but also any bladder abnormal structure. However, cystourethroscopy demonstrates a normal bladder without lesions in the early stage.
3.2. Clinicopathological characteristics
Histopathologically, the tumor tissues have plasmacytoid papillae or nest-like morphology, irregular distribution of acini and duct in matrices, with generalized sclerotic stroma which is associated with chronic inflammation. Besides, the no or slightly atypia small tumor cells are basophilic and infiltrate the prostate acinus and ducts. The nuclear polymorphism is obvious with high mitotic activity, with prominent basophilic nuclei [Citation7–10]. AR, PSA, PSMA, and PSAP are the common immunohistochemical markers of prostate cancer. At present, we have few insights into the marker of prostate urothelial carcinoma. CK7 and CK20 coexpression is a differential feature of urothelial carcinoma and 50–62% urothelial carcinoma is CK7 and CK20 coexpression [Citation11–13]. Therefore, CK7 and CK20 should be used as a first-line screen when primary UCP is considered. Besides, there are some other usual markers of the primary UCP, such as GATA3, 34βE12, CK5/6, P63, and uroplakim III, to help accurate diagnosis. GATA3, more than 85% urothelial are positive, as first-line markers of urothelial differentiation by the International Society of Urological Pathology (ISUP), is involved in the proliferation, differentiation, and development of urothelial cells, while negative in prostate cancer [Citation14]. 34βE12 expression rate is 65–97% in urothelial carcinoma, and the sensitivities are 65.2%, while CK5/6 is expressed in 75% of urothelial tumors [Citation9]. P63, as homologs tumor suppressor gene P53, is expressed in normal urothelium and high-grade urothelial neoplasms. Varinot et al. [Citation15] demonstrated P504S, CK7, CK20, and P63 positivity helped to confirm the primary UCP diagnosis.
3.3. Histological grade and stage
At present, the methods of grade and stage of the primary UCP differ from prostatic adenocarcinoma. The staging system for primary UCP is still under intense debate. Hardeman et al. [Citation16] presented the staging criteria according to tumor origin site and progression. The stage is as follows: Stage I: prostate primary ductal and urethral part carcinomas in situ. Stage II: tumor invasion into acinar tissue without break through the basal membrane. Stage III: tumor invasion into prostate stromal. Liedberg et al. [Citation17] were divided primary UCP into IVth stage according to tumor growth pattern: T1: tumor invasion into the subepithelial connective tissue. T2: tumor invasion into the prostate stromal, urethral corpus, or muscle of periurethral. T3: tumor invasion into corpus cavernosum penis, bladder neck, more than prostate. Grade is as follows: Grade I: well differentiation, Grade II: moderate differentiation, and Grade III: poorly differentiated [Citation16].
3.4. Diagnosis and differential diagnosis
The gold standard for diagnosing primary UCP is pathological examination. The diagnosis-positive rate is about 40% by transrectal needle biopsy while the transurethral prostatic resection with biopsy is 90% [Citation18]. The following diseases should be distinguished from primary UCP, such as prostate adenocarcinoma and secondary prostatic urothelial carcinoma. Immunohistochemistry is useful to distinguish primary UCP from prostate adenocarcinoma. Prostate adenocarcinoma marks, such as NKX3.1, PSA, and urothelial marker, such as 34βE12, P63, GATA3, and thrombomodulin can distinguish them [Citation19]. The sensitivity for diagnosing prostate adenocarcinoma of PSA and NKX3.1 are 100% and 88.3%, respectively, while the 34βE12, P63, GATA3, and thrombomodulin are 1.8%, 0%, 0%, and 0%, respectively. The sensitivity for diagnosing primary UCP of PSA and NKX3.1 is 9.4% and 0%, respectively, while the 34βE12, P63, GATA3, and thrombomodulin are 75.4%, 73.9%, 45.7%, and 84.8%, respectively [Citation20]. Primary UCP often presents with atypical symptoms, predominantly exhibited lower urinary tract obstruction symptoms, and is often misdiagnosed as benign prostate hyperplasia. Many patients may have been detected incidentally during transurethral prostate resection. The secondary prostate UCP frequently presents with gross hematuria also present with difficulty in urination or urinary retention due to the blockage urethral by clot. Cystoscopic examination can find the primary lesion located in the bladder. If no abnormality, a random biopsy should be performed to rule out carcinoma in situ in the bladder. Conversely, if prostate lesions were detected, urethrocystoscope should be performed.
3.5. Treatment
Primary UCP does not have a consensus on its optimal treatment. Principles of treatment of primary UCP are assumed to be the same as those for bladder urothelial carcinoma [Citation3]. Some scholars have suggested that radical prostatectomy is the most effective method to improve the survival outcome of localized primary UCP [Citation21]. Some also scholars believe that transurethral resection of the prostate and intravesical instillation of BCG postoperative should be taken into account when the tumor is limited to the mucosal layer or periurethral ducts, while radical prostatectomy should be taken when prostatic stromal invasion [Citation22]. Still, other scholars thought that surgery alone does not change the tumor progress of primary UCP, and surgery combined with chemotherapy may lead to better outcomes. Hamasaki et al. [Citation23] reported T3N2M0 patient, who underwent neoadjuvant chemotherapy combined with radical prostatectomy and retroperitoneal lymph node dissection and the histological examination of the tumor specimen postoperatively showed negative resection margins, had no recurrence and metastatic in the follow-up period of 3 years and 5 months. Notably and different to other tumor types, primary UCP, as a non-hormone dependent tumor, androgen deprivation therapy has been ineffective [Citation24]. Approximately, 50% of new patients with primary UCP in diagnosis presented with Stage T3 or T4 disease, and approximately 20% of patients have distant metastasis to the bone, lung, and liver because of its high degree of malignancy and strong invasiveness [Citation25]. For patients that are unable to undergo radical surgery, radiotherapy could control locally metastatic lesions, but the 5-year survival rate only about 34%, combined multimodality therapy with both chemotherapy and radiotherapy may be improve its survival time. Overall, UCP without infiltrated survival rate could reach 100% after radical cystoprostatectomy while a 5-year survival rate is only about 45% when prostatic epithelial or urethral invasion [Citation26]. Because lack of multicenter retrospective studies with large sample sizes, it needs to be confirmed whether neoadjuvant or adjuvant chemotherapy after surgery can improve the prognosis, but it is universally acknowledged that early diagnosis and radical resection benefit prognosis [Citation27].
4. Conclusion
The prostatic urothelial carcinoma is a very rare disease with a poor prognosis and an aggressive nature. Prostatic urothelial carcinoma should always be considered if the patient with severe lower urinary tract symptoms or hematuria, PSA, and digital rectal examination without abnormalities, without a personal history of urothelial cancer, but contrast-enhanced MRI showed the lesion located in the prostate. There is no universal agreement regarding how patients with UCP should be treated at present. As of right now, radical surgical resections remain the most effective treatment. The effectiveness of neoadjuvant or adjuvant chemotherapy is still controversial.
Ethical approval and consent to participate
The family patient’s informed written consent was obtained for the publication of this manuscript and any accompanying images.
Consent to publish
Written informed consent was obtained from the family of the patient for the publication of this manuscript and any accompanying images.
Author contribution
Design and drafting of the study: LL, FS, YX, and QW. Data curation: PZ, XY, and DW. Revision of the article: LL, FS, and QW. All authors have read and approved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Shen SS, Lerner SP, Muezzinoglu B, et al. Prostatic involvement by transitional cell carcinoma in patients with bladder cancer and its prognostic significance. Hum Pathol. 2006;37(6):726–734. doi: 10.1016/j.humpath.2006.01.027.
- Ende N, Woods LP, Shelley HS. Carcinoma originating in ducts surrounding the prostatic urethra. Am J Clin Pathol. 1963;40(2):183–189. doi: 10.1093/ajcp/40.2.183.
- Kisa E, Semiz HS, Küçük Ü, et al. Metastatic primary urothelial carcinoma of the prostatic urethra: a case report. Urol J. 2019;86(3):161–164. doi: 10.1177/0391560318808631.
- Tan MO, Tuncel A, Deniz N, et al. Case report: incidental primary transitional cell carcinoma of the prostate treated with transurethral prostatectomy only. Tumori J. 2003;89(4):440–442. doi: 10.1177/030089160308900419.
- Karpas CM, Moumgis B. Primary transitional cell carcinoma of prostate gland: possible pathogenesis and relationship to reserve cell hyperplasia of prostatic periurethral ducts. J Urol. 1969;101(2):201–205. doi: 10.1016/s0022-5347(17)62312-7.
- Filter ER, Gabril MY, Gomez JA, et al. Incidental prostate adenocarcinoma in cystoprostatectomy specimens: partial versus complete prostate sampling. Int J Surg Pathol. 2017;25(5):414–420. doi: 10.1177/1066896917696745.
- Sanguedolce F, Russo D, Mancini V, et al. Morphological and immunohistochemical biomarkers in distinguishing prostate carcinoma and urothelial carcinoma: a comprehensive review. Int J Surg Pathol. 2019;27(2):120–133. doi: 10.1177/1066896918814198.
- Moschini M, Soria F, Susani M, et al. Impact of the level of urothelial carcinoma involvement of the prostate on survival after radical cystectomy. Bladder Cancer. 2017;3(3):161–169. doi: 10.3233/BLC-160086.
- McKenney JK, Amin MB. The role of immunohistochemistry in the diagnosis of urinary bladder neoplasms. Semin Diagn Pathol. 2005;22(1):69–87. doi: 10.1053/j.semdp.2005.11.005.
- Kunju LP, Mehra R, Snyder M, et al. Prostate-specific antigen, high-molecular-weight cytokeratin (clone 34betaE12), and/or p63: an optimal immunohistochemical panel to distinguish poorly differentiated prostate adenocarcinoma from urothelial carcinoma. Am J Clin Pathol. 2006;125(5):675–681. doi: 10.1309/V1RY-91NK-X5AR-W2Q5.
- Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it!. J Cell Physiol. 2010;222(1):42–49. doi: 10.1002/jcp.21943.
- Moschini M, Zamboni S, Mattei A, et al. Radical cystectomy in pathological T4a and T4b bladder cancer patients: is there any space for Sub stratification? Urol Int. 2019;102(3):269–276. doi: 10.1159/000493899.
- Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004;171(1):139–144. doi: 10.1097/01.ju.0000102302.26806.fb.
- Wu S, Lin SX, Lu M, et al. Assessment of 5-year overall survival in bladder cancer patients with incidental prostate cancer identified at radical cystoprostatectomy. Int Urol Nephrol. 2019;51(9):1527–1535. doi: 10.1007/s11255-019-02181-7.
- Varinot J, Cussenot O, Roupret M, et al. HOXB13 is a sensitive and specific marker of prostate cells, useful in distinguishing between carcinomas of prostatic and urothelial origin. Virchows Arch. 2013;463(6):803–809. doi: 10.1007/s00428-013-1495-0.
- Hardeman SW, Perry A, Soloway MS. Transitional cell carcinoma of the prostate following intravesical therapy for transitional cell carcinoma of the bladder. J Urol. 1988;140(2):289–292. doi: 10.1016/s0022-5347(17)41585-0.
- Liedberg F, Chebil G, Månsson W. Urothelial carcinoma in the prostatic urethra and prostate: current controversies. Expert Rev Anticancer Ther. 2007;7(3):383–390. doi: 10.1586/14737140.7.3.383.
- Bin Y, Guoliang W, Huiying H. Advances in pathology, diagnosis and treatment of nested variant urothelial carcinoma. Chin J Urol. 2017;38(2):155–157.
- Lin MC, Lin JJ, Hsu CL, et al. GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness. Oncogene. 2017;36(30):4243–4252. doi: 10.1038/onc.2017.8.
- Oh WJ, Chung AM, Kim JS, et al. Differential immunohistochemical profiles for distinguishing prostate carcinoma and urothelial carcinoma. J Pathol Transl Med. 2016;50(5):345–354. doi: 10.4132/jptm.2016.06.14.
- Taylor JH, Davis J, Schellhammer P. Long-term follow-up of intravesical bacillus Calmette-Guérin treatment for superficial transitional-cell carcinoma of the bladder involving the prostatic urethra. Clin Genitourin Cancer. 2007;5(6):386–389. doi: 10.3816/CGC.2007.n.021.
- Cui J, Wang W, Chen S, et al. Combination of intravesical chemotherapy and bacillus Calmette-Guerin versus bacillus Calmette-Guerin monotherapy in intermediate- and high-risk nonmuscle invasive bladder cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2016;95(3):e2572. doi: 10.1097/MD.0000000000002572.
- Hamasaki T, Kondo Y, Ogata Y, et al. Advanced carcinoma of the prostatic urethra in a patient with marked response to chemotherapy, leading to preservation of the bladder. Int J Clin Oncol. 2010;15(1):109–111. doi: 10.1007/s10147-009-0006-4.
- Xing J, Zhang C, Liu Z, et al. Primary urothelial carcinoma of the prostate: a case report. Chin J Urol. 2019;40(2):148–148.
- Oliai BR, Kahane H, Epstein JI. A clinicopathologic analysis of urothelial carcinomas diagnosed on prostate needle biopsy. Am J Surg Pathol. 2001;25(6):794–801. doi: 10.1097/00000478-200106000-00012.
- Lerner SP, Shen S. Pathologic assessment and clinical significance of prostatic involvement by transitional cell carcinoma and prostate cancer. Urol Oncol. 2008;26(5):481–485. doi: 10.1016/j.urolonc.2008.03.002.
- Palou J, Baniel J, Klotz L, et al. Urothelial carcinoma of the prostate. Urology. 2007;69(1l):50–61. doi: 10.1016/j.urology.2006.05.059.