Abstract
Objective
We evaluated change (Δ) in AMSS in men with adult-onset testosterone deficiency (TD) on/not on testosterone undecanoate (TU) by analysing a registry of men with adult-onset TD.
Methods
Analyses were performed using non-parametric statistics to determine ΔAMSS at 6–12 monthly intervals in men on/not on TU and movement in AMSS. Factors predicting ΔAMSS were established via linear/multiple regression.
Results
TU was significantly associated with lower AMSS values compared with that at baseline/prior assessment during the initial 42 months treatment; 259 of the 260 men showed improvement. In the 361 men not on TU, AMSS values increased during 60 months of follow-up compared with that at baseline/prior assessment; improvement after 60 months was evident in 1 man, whilst AMSS remained the same or worsened in 213 and 147 men, respectively. In men on TU, baseline AMSS was inversely associated with ΔAMSS (R2 = 0.97), with no other factors reaching significance. Baseline AMSS, age, serum total testosterone (TT), waist circumference (WC), and diastolic blood pressure (BP) were associated with ΔAMSS in men not on TU.
Discussion
We show that TU was associated with lower AMSS in men with adult-onset TD whilst non-treatment led to increased values. Baseline AMSS values inversely predicted ΔAMSS in both groups.
Introduction
Adult-onset testosterone deficiency (TD), a syndrome prevalent in 0.6–12% of men aged over 50 years, is diagnosed by the presence of low serum testosterone levels and related clinical features associated with low hormone levels, following exclusion of testicular and hypothalamic-pituitary dysfunction [Citation1,Citation2]. The Aging Male Symptoms Scale (AMSS) is a validated questionnaire proven to be useful in assessing health-related quality of life (QoL) symptoms of aging that include many of the classifying symptoms of adult-onset TD [Citation3–7]. Further, it also appears to be valuable in evaluating benefit following testosterone therapy (TTh) [Citation5,Citation7].
Diem et al. reviewed 38 randomized controlled trials (RCTs) of at least 6 months’ duration and 20 long-term longitudinal cohort studies evaluating TTh (c.f. placebo/no treatment) and showed improvements in sexual health and QoL indices [Citation8]. One of the limitations acknowledged by the authors was the relatively short follow-up of the RCTs, with very few exceeding 12 months [Citation8]. Similarly, Elliott et al. demonstrated TTh to be associated with improved QoL indices in an analysis of 23 RCTs with treatment duration in excess of 3 months, although moderate/severe depression attenuated the relationship [Citation9]. The median follow-up of the RCTs studied was 6 months and it was accepted that a longer treatment duration may have been required for alleviation of some of the symptoms [Citation9]. Further, both analyses included studies using different testosterone preparations and varying QoL outcomes measures [Citation8,Citation9]. Accordingly, we believe it is important that a long-term study assessing QoL periodically at fixed time points using a single measure (AMSS) following TTh (using a single preparation) would limit study design heterogeneity.
Saad et al., using data from 2 registries, reported significant improvement in QoL evaluated via AMSS in 411 men with adult-onset TD and a body mass index (BMI) ≥ 30 kg/m2 when treated with testosterone undecanoate (TU) over a mean period of 6 years [Citation10]. AMSS improved significantly in subgroups stratified by BMI; mean ± standard error values of change (Δ) in AMSS in men with BMI of 30–34.9 kg/m2, 35–39.9 kg/m2, and ≥40.0 kg/m2 were −32.48 ± 0.7 (mean baseline AMSS ± standard deviation (SD) = 53.3 ± 10.05), −33.39 ± 0.7 (mean baseline AMSS ± SD = 52.59 ± 9.43), and −38.24 ± 1.16 (mean baseline AMSS ± SD = 57.0 ± 9.88), respectively [Citation10].
In this study, we further analyse individual patient data from one of the 2 registries studied by Saad et al. now comprising more patients with longer follow-up. The other registry ceased being active in 2016 [Citation10,Citation11]. After initially studying ΔAMSS values at 6 monthly intervals in men (on/not on TU) with adult-onset TD, we established factors that predicted ΔAMSS. We then established if there was an association between baseline AMSS (at initial assessment prior to TTh being offered) and ΔAMSS (also including metabolic parameters associated with change during TTh in the statistical models) in accordance with the Wilder Principle that baseline values predicted response following treatment (law of initial value) [Citation3,Citation12,Citation13]. Finally, we determined changing distribution of AMSS categories (based on clinical symptoms) in men on/not on TU.
Materials and methods
The observational, analysis of an ongoing cumulative registry database comprised data on 737 men (men with primary hypogonadism and Klinefelter syndrome were excluded from the database) with sexual dysfunction, other classifying symptoms of adult-onset TD symptoms and lower urinary tract symptoms with baseline serum total testosterone (TT) ≤12.1 nmol/L [Citation11]. Details of the registry protocol, methodology, subsequent clinical, and laboratory assessments (carried out at least 6 monthly) and list of publications has been previously published [Citation11]. All 737 men were offered TTh; 353 men opted for parenteral TU, 1000 mg/12 weeks following a 6-week interval between first and second administration (median (IQR) follow-up = 105 (78–141) months), whilst the remaining 384 men opted against treatment for financial or safety concerns (median (IQR) follow-up = 114 (96–126) months). The men opting against TTh was against medical advice provided by AH and related to reluctance to self-fund TTh (some insurances were reluctant to cover TTh). Further, it was evident that much of the reluctance related to primary care warning against TTh. This included erroneous warnings regarding prostate cancer, adverse effects on liver function and cardiovascular function [Citation11]. Treatment for urological conditions such as benign prostatic hypertrophy, lower urinary tract symptoms and erectile dysfunction was provided to all the men on the registry by AH, whilst non-urological conditions (e.g. diabetes, hypertension, dyslipidemia, etc.) were managed in primary care.
The number of men on/not on TU used principally in this study with ΔAMSS data at 60 months were 260 and 361, respectively. The study was conducted in accordance with the ethical guidelines for observational studies laid out by the German Medical Association. After receiving a detailed and informative explanation regarding the nature and the purpose of the study including the intention to publish in an anonymised fashion, all subjects provided written consent, regardless of whether or not they opted for TTh. Following review, Ethics Committees in Germany and England stated that formal approvals of this analysis of the registry were not required (details of the correspondence and copy of the Institutional Review Board Statement (IRBS14032024 – University Hospitals Birmingham NHS Foundation Trust) can be obtained from the corresponding author).
Serum TT was estimated using the Alinity i-Module, Architect intra-assay (Abbott Diagnostics) whilst glycaemic status via hemoglobin A1c (HbA1c) was measured using high pressure liquid chromatography (TOSOH, HLC-723 series). At every assessment, blood pressure (BP) was checked at least twice, following the initial BP measurement TU was administered and BP determined again. In the event of differences in BP readings being < 5 mmHg, a mean value was recorded, if BP readings varied by > 5 mmHg a further check was carried out and in the event of the difference <10 mm Hg the mean of the 2 closer values entered. If BP values differed by >10 mmHg, a 24-h BP monitoring was carried out, and the mean BP recorded.
Statistical analysis
AMSS values at every 6–12 monthly time-point during follow-up (up to 60 months) was compared with baseline AMSS (at initial assessment prior to TTh being offered) and the previous AMSS values via non-parametric sign-rank test in men on/not on TU (). As the baseline AMSS value differed between the groups (p < 0.0001, rank-sum test) we did not directly compare the men opting for TU with those opting against it and mainly confined the statistics to intra-group analyses. Differences in age, waist circumference (WC), HbA1c, BP, and sexual function scores were evident between the groups with the values worse in the men opting for TTh as we have previously shown [Citation11]. We can speculate that the men with worse symptoms associated with adult-onset TD opted for TTh.
Table 1. AMSS and ΔAMSS values in men on/not on TU at 6-monthly follow-up intervals.
We then carried out linear/multiple regression analyses with ΔAMSS as the outcome with baseline AMSS as the independent variable studied ( and ), with potential confounders (this was based on factors that varied between the two groups, as changes in serum TT, WC, HbA1c, and BP [Citation11] could perhaps have affected AMSS values) added to subsequent regression models (). Finally, we categorized AMSS values (baseline and after 60 months) into symptom-defining categories () and determined the number of men moving from one category to another during the follow-up in men on/not on TU.
Figure 1. Graphical illustration of the association (based on – Model 1) between ΔAMSS after 60 months and baseline AMSS values.
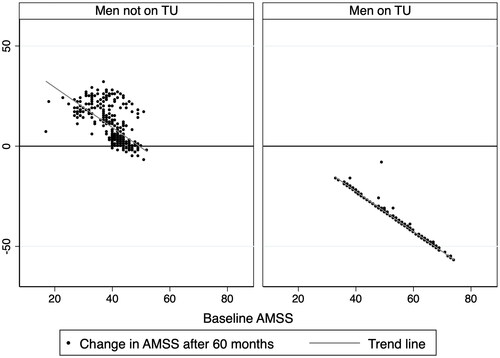
Figure 2. A grid of the number of men in AMSS at baseline and 60 months follow-up, categorized into symptoms-based subgroups. AMSS categories by symptoms associated with low testosterone levels. 17–26: no significant symptoms; 27–36: mild symptoms; 37–49: moderate symptoms; >49: severe symptoms.
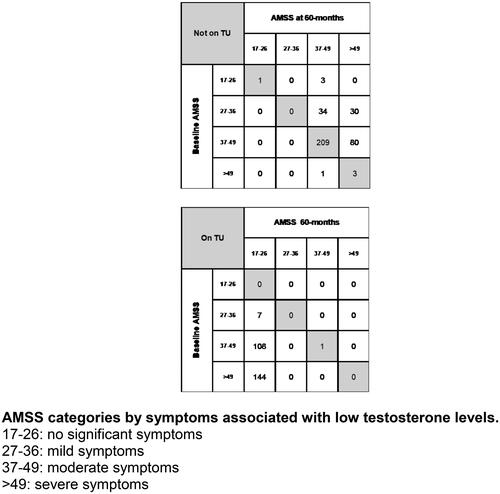
Table 2. Results from linear/multiple regression models with ΔAMSS after 60 months as the dependent variable and baseline AMSS and other metabolic factors as independent variables – separate models were carried out in men on/not on TU.
Results
AMSS and Δ AMSS values during follow-up
As recruitment into the registry was ongoing, the number of men at each assessment decreased (). AMSS and Δ AMSS values at baseline and at every time point during follow-up is shown in . In men on TU, AMSS values decreased (improved QoL) significantly (p < 0.0001, sign-rank) at every time point compared to the baseline value. Further, in this group of men the AMSS values decreased significantly compared to that recorded 6 months previously, up to 42 months. No significant differences were observed between AMSS values between 48 and 42 months (p = 0.68, sign-rank) as well as 60 and 48 months (p = 0.78, sign-rank). Hence, we did not extend the analyses in both men on/not on TU to time points > 60 months. also shows that improvement in AMSS was observed in every single man after 60 months on TU compared to baseline. In the complementary group of men opting against TU, the AMSS values increased (worsening QoL) at every time point compared to both baseline and the previous recorded AMSS values ().
Factors predicting ΔAMSS values after a follow-up of 60 months
Separate linear regression analyses (, Model 1) were carried out with ΔAMSS as the dependent variable and baseline AMSS as the independent variable in men on/not on TU. Baseline AMSS was inversely associated with ΔAMSS in both groups of men (p < 0.001) with identical coefficients (−1.00, , Model 1), although the 95% confidence intervals (CIs) were wider in the men not on TU. In the men on TU, the R2 value of 0.97 suggested that nearly all the variability was explained by the above association and is shown in . This association remained significant (p < 0.001) even when the models were adjusted for age, serum TT, WC, HbA1c, and BP values recorded after 60 months of treatment, both individually (, Models 2–7) and concurrently (, Model 8).
Although the association between ΔAMSS and baseline AMSS remained significant (p < 0.001) in men not on TU even when adjusting for age, serum TT, WC, HbA1c, and BP values (, Models 2–7), each of these factors were also seen to be significantly associated with ΔAMSS. In the association between ΔAMSS and baseline AMSS in men not on TU (, Model 1), the R2 value was lower (0.40) and hence visually demonstrates greater scatter than in men on TU. When all the factors were entered into a single model (, Model 8), baseline AMSS, age, and serum TT were inversely associated with ΔAMSS whilst WC, and diastolic BP were positively associated with ΔAMSS in the men not on TU.
Change in AMSS categories based on symptoms after 60 months
All men were stratified by AMSS categories at baseline and after 60 months of follow-up based on symptoms; 17–26 (no significant symptoms), 27–36 (mild symptoms), 37–49 (moderated symptoms), and >49 (severe symptoms). The patient numbers within each category at baseline and after 60 months of follow-up is shown (men on/not on TU illustrated as a grid) in . At baseline, none of the 260 men on TU and only 1 of the 361 men not on TU (patient numbers restricted to those with AMSS values at baseline and 6 months of follow-up) had AMSS values between 17 and 26 (). A lower AMSS category (improvement in symptoms) was evident in 259 of the 260 men on TU after 60 months, with the remaining individual staying within the same category, even though the AMSS after 6 months decreased from 49 to 41 (, shaded diagonal grid box and footnote). Contrastingly, in the 361 men opting against TU, a lower AMSS (improvement in symptoms) category was only evident in 1 man (AMSS decrease from 51 to 44), whilst 213 men stayed within the same category, and the remaining 147 men moved to a higher (worsening of symptoms) category ( and footnote).
Discussion
Our analyses of this ongoing registry showed that TU was associated with significantly lower AMSS values (improved QoL) in men with adult-onset TD, compared to baseline AMSS and AMSS values recorded 6 months previously. This improvement continued up to 42 months follow-up. There was no significant change in the AMSS values subsequently (42–48 months and 48–60 months). Of the 260 men on TU, 259 men demonstrated improved AMSS categories based on symptoms: 3 category improvement in 144 men, 2 category improvement in 108 men, and a single category improvement in 7 men. All the 259 men achieved a AMSS category of 17–26 (no significant symptoms). Only a single man remained with the same AMSS category, even though the AMSS value decreased from 49 to 41. A different pattern was observed in the men not on TU, with AMSS values increasing after 60 months compared to baseline AMSS and AMSS values recorded at the prior assessment. Of the 361 men not on TU, improvement in AMSS category after 6 months was only evident in 1 man, whilst 213 men remained in the same AMSS category, and 147 men transferred to a worse AMSS category (114 and 33 men worsened by 1 and 2 categories, respectively).
In men on TU, baseline AMSS values were significantly inversely associated with ΔAMSS with a near perfect R2 value of 0.97 demonstrating the strength of the association. None of the other factors (age, serum TT, WC, HbA1c, and BP) included in the regression models were significantly associated with ΔAMSS. ΔAMSS after 60 months of TU treatment was in accordance with the Wilder law of initial value [Citation12]. This phenomenon was also observed by Heinemann et al. following TTh for 3 months using gel preparations [Citation3].
Although baseline AMSS was significantly associated with the outcome of ΔAMSS in men not on TU, other factors including age, serum TT, WC, and diastolic BP at 60 months follow-up were also related to the outcome. This could be due to differing trajectories of factors in the 2 groups (on/not on TU) as previously reported using the same registry [Citation13,Citation14]. In men on TU in our study after 60 months follow-up, age (p < 0.0001), serum TT (p < 0.0001), WC (p < 0.0001), HbA1c (p = 0.028), systolic BP (p < 0.0001), and diastolic BP (p < 0.0001) significantly differed (rank-sum) from those not on TU. Median (IQR) values at 60 months follow-up for age, serum TT, WC, HbA1c, systolic BP, and diastolic BP were 65 (60, 69) years, 17.7 (15.9, 19.4) nmol/L, 98 (95, 103) cm, 6.2 (5.4, 6.5)%, 132.5 (127, 140) mm Hg, and 77 (75, 81.5) mm Hg, respectively, in men on TU. The corresponding median (IQR) values for age, serum TT, WC, HbA1c, systolic BP, and diastolic BP were 69 (65, 72) years, 9.7 (8.7, 10.1) nmol/L, 109 (103, 116) cm, 5.9 (5.3, 8.0)%, 138 (132, 153) mm Hg, and 79 (75, 90) mm Hg, respectively, in men not on TU. Thus, we can speculate that worsening levels of these factors after 60 months follow-up, evident in men not on TU, perhaps led to their associations with ΔAMSS and perhaps contributed to worsening values. Interestingly, the inverse association between baseline AMSS and ΔAMSS suggested that the increase (worsening) in AMSS was greater in men with lower baseline values.
Depression and anxiety have been seen to affect AMSS. Lee et al. in a study of 176 men showed significant correlations between AMSS and depression/anxiety evaluated using the Hospital Anxiety and Depression Scale – Depression (HADS-D) questionnaire [Citation15]. Hackett et al. demonstrated an improvement in AMSS values following TU treatment for 30 weeks compared to placebo in men with type 2 diabetes and low serum testosterone levels, with this improvement only evident in men with no clinically significant depression (HADS-D < 11) [Citation16]. Unfortunately, we did not have HADS-D values in our dataset as it would have been useful to study the rate of change in AMSS () and predictive associations () in the cohort stratified by HADS-D. However, the R2 of 0.97 in the men on TU, suggests that factors other than baseline AMSS have a limited role in predicting ΔAMSS at 60 months, this perhaps due to a longer duration of TTh than in the RCT by Hackett et al. [Citation16] Although, baseline AMSS was significantly (c: −0.97, 95% CI: −1.03, −0.90), p < 0.001, n = 346) associated with ΔAMSS after 6 months of TU (the time point closest to the report by Hackett et al.) [Citation16], the R2 value was lower at 0.69. Thus, it could be that ΔAMSS rate at early stages of TU treatment could be influenced by factors in addition to baseline AMSS.
Our study has strengths and weaknesses. In this longitudinal registry study, in contrast to an RCT, the study design did not permit exclusion/inclusion factors. Further, no blind randomization took place as all the men were offered TU, with take-up based on perception of TTh safety and finance. This led to significant differences in baseline values in the men on/not on TU and a likelihood of selection bias. We have previously shown men opting for TU differed in baseline factors (e.g. WC, glycaemic control, dyslipidaemia, and hypertension) compared to the men opting against treatment [Citation14]. Hence, we mainly restricted the statistics to intra-group analyses. It is important to emphasize our observations to TTh with TU. Further, the associations that we have reported do not indicate causation. More studies including RCTs with long follow-up periods are required as there is a possibility of regression toward the mean, although the observed Wilder effect does not suggest this [Citation12]. Unfortunately, we did not have HADS-D values which have previously shown to affect ΔAMSS [Citation16] and CAG repeat numbers in exon 1 of the androgen receptor gene, influencing receptor sensitivity [Citation17] which would have allowed us to study the velocity of ΔAMSS in both men on/not on TU. We have recently shown that 30-week of TU/lifestyle advice improves somatic, psychological, and sexual subscale values that makeup the summation AMSS scores, whilst placebo/lifestyle advice only led to improvement in the somatic subscale values [Citation18]. It would have been interesting to analyse the individual subscale data in this study with a longer follow-up to see if changes in the subscale scores were associated with treatment duration.
Conclusion
TU treatment over 60 months in men with adult-onset TD resulted in every man demonstrating improved AMSS values with all significant improvement evident after 42 months of treatment. The AMSS values worsened in the complementary group of men not on TU, with the AMSS values continuing to increase for at least 60 months. Baseline AMSS values predicted ΔAMSS inversely in both groups; in men on TU higher, baseline AMSS values led to greater decrease (improvement of symptoms and QoL) during follow-up, whilst in men not on TU, lower baseline AMSS values led to a greater increase (worsening of symptoms and QoL). As AMSS is considered a measure of QoL we would recommend that it is monitored periodically in men with adult-onset TD and evaluated as an outcome in men opting for and against TTh. Obviously, our results need to be validated with identification of factors that may affect rate of ΔAMSS at different time points.
Author contributions
SR, PR, and CSK: design of study, data analysis, preparation of manuscript.
AH, KSH, and FS: patient recruitment, data collection, preparation of manuscript.
PD: transposing the data, maintaining the database.
MZ, GH, AM and RCS: preparation of manuscript.
Disclosure statement
The authors have no competing interests to declare that are relevant to the content of this article.
Data availability statement
All reasonable requests for data should be directed to corresponding author.
Additional information
Funding
References
- Antonio L, Wu FCW, Moors H, et al. Erectile dysfunction predicts mortality in Middle-aged and older men independent of their sex steroid status. Age Ageing. 2022;51(4):afac094.
- Hackett G, Kirby M, Rees RW, et al. The british society for sexual medicine guidelines on male adult testosterone deficiency, with statements for practice. World J Mens Health. 2023;41(3):e33–537. doi: 10.5534/wjmh.221027.
- Heinemann LA, Saad F, Zimmermann T, et al. The aging males’ symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1(1):15. 1 doi: 10.1186/1477-7525-1-15.
- Akehi Y, Tanabe M, Yano H, et al. A simple questionnaire for the detection of testosterone deficiency in men with late-onset hypogonadism. Endocr J. 2022;69(11):1303–1312. doi: 10.1507/endocrj.EJ22-0073.
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male. 2015;18(1):5–15. doi: 10.3109/13685538.2015.1004049.
- Morley JE, Perry HM, 3rd, Kevorkian RT, et al. Comparison of screening questionnaires for the diagnosis of hypogonadism. Maturitas. 2006;53(4):424–429. doi: 10.1016/j.maturitas.2005.07.004.
- Moore C, Huebler D, Zimmermann T, et al. The aging males’ symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46(1):80–87. doi: 10.1016/j.eururo.2004.01.009.
- Diem SJ, Greer NL, MacDonald R, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American college of physicians. Ann Intern Med. 2020;172(2):105–118. doi: 10.7326/M19-0830.
- Elliott J, Kelly SE, Millar AC, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open. 2017;7(11):e015284. doi: 10.1136/bmjopen-2016-015284.
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond). 2016;40(1):162–170. doi: 10.1038/ijo.2015.139.
- Haider KS, Haider A, Doros G, et al. Design and conduct of a real-world single center registry study on testosterone therapy in men with hypogonadism. Androgen Clin Res Ther. 2021;2(1):1–17. doi: 10.1089/andro.2020.0011.
- Messerli FH, Bangalore S, Schmieder RE. Wilder’s principle: pre-treatment value determines post-treatment response. Eur Heart J. 2015;36(9):576–579.
- Traish AM, Haider A, Haider KS, et al. Long-Term testosterone therapy improves cardiometabolic function and reduces risk of cardiovascular disease in men with hypogonadism: a Real-Life observational registry study setting comparing treated and untreated (control) groups. J Cardiovasc Pharmacol Ther. 2017;22(5):414–433. doi: 10.1177/1074248417691136.
- Hackett G, Mann A, Haider A, et al. Testosterone replacement therapy. Effects on blood pressure in hypogonadal men. World J Mens Health. 2024 [cited 2024 Feb 14].
- Lee CP, Jiang JR, Chen Y, et al. The "aging males’ symptoms" (AMS) scale assesses depression and anxiety. Aging Male. 2013;16(3):97–101.
- Hackett G, Cole N, Bhartia M, et al. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. J Sex Med. 2013;10(6):1612–1627. doi: 10.1111/jsm.12146.
- Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92(10):3844–3853. doi: 10.1210/jc.2007-0620.
- Ramachandran P, Zitzmann M, König CS, et al. Testosterone undecanoate is associated with improved ageing male symptoms score in men with type 2 diabetes and adult-onset testosterone deficiency: re-analyzed results from a randomised controlled trial. Explor Endocr Metab Dis. 2024. (in press).
