Abstract
The effect of paternal age on fertility remains unclear. This retrospective study aims to examine the impact of male age on semen parameters and the reproductive outcomes of men admitted to an infertility center over a 9-year period. A total of 8046 patients were included in the study. Men were divided into four age groups. The groups were evaluated for semen parameters and reproductive outcome. The 21–30 year group presented lower sperm concentrations in comparison to those aged 31–40 and 41–50, yet shared a similar concentration to those over 50 years of age. Moreover, grades A and B decreased significantly in men aged over 50 years. The highest progressive motility and normozoospermia were observed in the age group 31–40 years while men over 50 years of age had the highest rates of asthenozoospermia and oligoasthenozoospermia. Furthermore, live birth results were reported in 5583 of the patients who underwent intracytoplasmic sperm injection (ICSI) and were found highest between 31–40 years of age. To our knowledge, this is the largest study in Turkey focusing on male age-related semen parameters and ICSI pregnancy outcomes. The study demonstrates that age is a significant factor for semen quality and live birth.
1. Introduction
Fertility preservation has recently gained importance in the aging process, with the increase in average human life expectancy and the advancement of the childbearing age. Female fertility decreases with a sharp decline in ovarian reserve after the age of 35. Fertility in women usually diminishes with the onset of menopause, around age 45–50 [Citation1–5]. However, men can father children after the age of 45–50 years as no critical age threshold for the production of sperm has been reported. Nevertheless, debate continues on whether advanced paternal age is associated with a decrease in semen quality and eventually a higher risk of infertility [Citation3,Citation6–8]. Advanced paternal age is especially common in developed countries due to higher socioeconomic status and the improved success rates and availability of assisted reproductive techniques (ART), which enable couples to delay childbearing [Citation9, Citation10].
Semen quality is a crucial indicator of male reproductive function, as determined through semen analysis. This assessment evaluates sperm count, morphology, and motility, providing a fundamental evaluation of male fertility [Citation11]. The best values for semen quality, according to the WHO’s criteria, are observed between 30 and 35 years of age, with the most significant decline occurring after 55 years of age [Citation2, Citation3]. Any deterioration in semen quality may decrease fertility rates and increase the use of ART [Citation12, Citation13]. A growing body of evidence indicates that advanced paternal age is associated with a delay in conception and subfecundity, as well as an increased risk of spontaneous abortions, thereby increasing the risk of natural reproductive failure [Citation14]. Furthermore, several recent works have demonstrated that advanced paternal age results in male infertility and poor ART outcomes [Citation15, Citation16]. On the contrary, there are also studies reporting no significant change in sperm parameters and ART success as men get older [Citation15, Citation17, Citation18].
This study aims to investigate the relationship between paternal age and semen parameters, namely sperm concentration and progressive motility, and to explore the possible effect of paternal age on pregnancy and live birth.
2. Participants and methods
2.1. Study population
The medical records of infertile couples at Acıbadem Altunizade IVF Center between 2010 and 2019 were reviewed in this retrospective study. The study included 8046 men admitted to IVF center who underwent semen analysis regardless of the cause of infertility including female infertility, male infertility or unexplained infertility. The exclusion criteria for this study included individuals who had experienced infections in the last 6 months, those who had previous treatment with chemotherapeutic agents or radiotherapy, and those who had testicular tumors. The age range of the men in the study was 21 to 65. Participants were divided into four age groups as follows: 21–30 years, 31–40 years, 41–50 years, and over 50 years of age. Semen parameters and resulting pregnancy and live birth rates were included as clinical information. All participants included in this study were informed and their written informed consent was obtained both for participation and publication. The study protocol was approved by the Non-Interventional Ethics Committee of Maltepe University (approval date: 27.04.2023, approval number: 2023/09-19).
2.2. Semen analysis
Semen samples were collected after 3–5 days of sexual abstinence. Microdissection testicular sperm extraction (micro-TESE) was performed in men diagnosed with azoospermia. All samples were processed for semen analysis in the same laboratory and under the same conditions. Study data collected between 2010 and 2019 were retrospectively analyzed in accordance with the World Health Organization (WHO) 2021 criteria ().
Table 1. WHO semen analysis normal values, 2021.*
2.2.1. Sperm concentration
Samples collected in a sterile container were analyzed after a 10–30 min of liquefaction at 37 °C in an incubator. To determine sperm concentration, 10 μL of semen samples were placed in a Makler counting chamber and counted under a phase contrast microscope with a 20X objective.
2.2.2. Sperm motility
Sperm motility is examined in 4 categories: grade A, which refers to fast forward movement; grade B, which refers to slow forward movement; grade C, which refers to sluggish movement; and grade D, which refers to immotility. Sperm progressive motility is the sum of grade A and grade B. Motility evaluation was performed by counting at least 100 spermatozoa and the results were expressed as percentage (%).
2.2.3. Classification of participants
The participants were classified based on their sperm concentration as normozoospermic (sperm concentration more than 16 × 106/mL), oligozoospermic (sperm concentration less than 16 × 106/mL), or azoospermic (no spermatozoa in the ejaculate), and based on their motility values as asthenozoospermic (<30% progressive motility), or oligoasthenozoospermic (sperm concentration less than 16 × 106/mL and <30% progressive motility) according to WHO 2021 criteria.
2.3. ICSI and embryo transfer
Spermatozoa were immobilized and injected into mature oocytes using a micromanipulator. Fertilization was evaluated 12–18 h after ICSI by the presence of two pronuclei and two polar bodies. Fresh or frozen embryo transfer was performed depending on the study protocol in appropriate cases. Fresh embryo transfer was performed on day 3–5. Since many studies comparing pregnancy outcomes in cases of frozen-thawed embryo transfer and fresh embryo transfer showed no differences in pregnancy rates, all cases of fresh and frozen-thawed embryo transfer were included in the study [Citation19–21]. In accordance with the single and conditional two embryo transfer regulations in Turkey [Citation22], only one embryo was transferred when the woman’s age was below 35 years, while a maximum of two embryos were transferred in patients aged 35 years and over.
2.4. Pregnancy evaluation
A total of 5583 couples undergoing embryo transfer after ICSI were evaluated for pregnancy. Serum β-HCG levels were evaluated 10 to 12 days after the embryo transfer and the gestational sac was examined by transvaginal ultrasound at 4–6 weeks. Pregnancy was considered positive if the serum β-HCG level was above 10 mIU/ml.
2.5. Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM SPSS Statistics, Armonk, NY, USA) was used for statistical analyses. Data are presented as mean ± standard deviation (SD) or median and min-max for quantitative variables. Data are presented as a % with n for qualitative variables. The assessment of normality was performed with the Kolmogorow-Smirnov and Shapiro-Wilk tests. Comparisons of the four groups were performed with the Kruskal–Wallis and Tamhane’s post hoc test. In order to facilitate intergroup comparisons, the 21–30 and 31–40 age groups were selected as the reference age groups. Multivariate analysis of variance (MANOVA) with Tamhane post hoc test and the Pearson Chi-Square with Bonferroni post hoc tests were used to compare pregnancy and live birth rates. Differences were considered statistically significant when p < 0.05.
3. Results
A total of 8046 male partners of infertile couples were included in the study during the 9-year study period. The descriptive characteristics are depicted in . The average male age and sperm concentration were 37.39 ± 6.16 years and 31.28 ± 28.17 (106/ml), respectively. The average maternal age was 34.47 ± 5.49 years. Men aged 31–40 years old accounted for the largest portion (60%), while men aged 21–30, 41–50 and over 50 years of age corresponded to 12%, 25% and 3% of the study population, respectively. Similarly, women aged 31–40 years old accounted for the largest portion (58.4%), while women aged 20–30, and 41–50 years of age corresponded to 31.9% and 9.7% of the study population, respectively. The study included 5583 ICSI cycles and a 56.6% (n = 3162) pregnancy rate.
Table 2. Descriptive characteristics of 8046 cases. The data are shown as mean ± standard deviation (SD) or % and number (n).
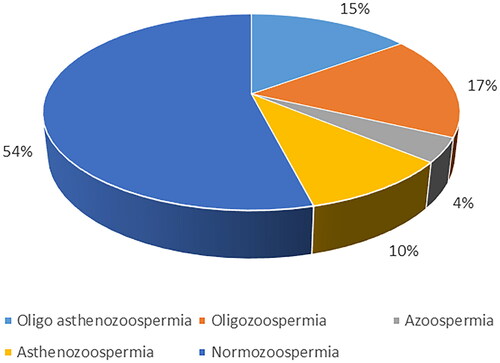
Normozoospermia was identified in 54% of the study population, while the rest of the samples were associated with the following abnormalities: oligozoospermia (17%), oligoasthenozoospermia (15%), azoospermia (4%), and asthenozoospermia (10%) ().
Figure 1. Pie chart demonstrating the distribution of men with normozoospermia, oligozoospermia, oligoasthenozoospermia, azoospermia, and asthenozoospermia based on the sixth edition of the WHO laboratory manual for the examination and processing of human semen, 2021.
Increase in maternal age was parallel with paternal age (). While the mean maternal age was 27.91 ± 4.14 for men aged 21–30 years, it was 38.69 ± 4.21 for men over 50 years (p = 0.000). The mean maternal ages were similar for men aged 31–40 years and 41–50 years (33.77 ± 4.51 vs. 33.78 ± 4.51, p = 1.0). A comparison of the semen characteristics among different age groups is demonstrated in . The mean values for sperm concentration and progressive motility were higher than the lowest reference limits set by the WHO in 2021 in all age groups. Higher sperm concentrations were found in men aged 31–40 and 41–50 in comparison to those aged 21–30 (p = 0.000 and p = 0.005, respectively). On the other hand, similar sperm concentrations were observed between men aged 21–30 and over 50 years of age (p = 0.997).
Table 3. Comparison of semen parameters, pregnancy and live birth rates according to the paternal age.
The sperm grade A values were similar between the age groups 21–30 and 31–40 (p = 0.905), while a progressive decline was observed with increasing male age (p = 0.002 for the age group 41–50 and p = 0.000 for men over 50 years of age in comparison to those aged 21–30) (). A similar trend was observed for grade B. The age groups 41–50 and over 50 years of age displayed lower grade B values, compared to the age group 31–40 (p = 0.039 and p = 0.000, respectively). As for progressive motility, this was highest in men in the 31–40 age group (p = 0.000, p = 0.034, and p = 0.005 in comparison to the age groups 21–30, 41–50, and over 50 years, respectively).
Interestingly, the 21–30 age group demonstrated a higher rate of azoospermia in comparison to the age groups 31–40 and 41–50 (p = 0.000 and p = 0.000, respectively). Asthenozoospermic and oligoasthenozoospermic men were observed most frequently in men over 50 years of age, though a significant increase was observed in all age groups in comparison to the 21–30 year age group (p = 0.000, p = 0.000, and p = 0.000 in men aged 31–40, 41–50, and over 50 years, respectively). Normozoospermia and oligozoospermia were least frequently observed in men over 50 years of age (p = 0.000) when compared to the other age groups.
Both MANOVA and the Pearson Chi-Square analysis with respective post hoc tests resulted in similar pregnancy rates among all the age groups (p = 0.741). Live births were most frequently observed in the age group 31–40, with a significant decrease in the 41–50 and 50+ age groups in comparison to the 21–30 age group (p = 0.000, p = 0.004, p = 0.001 respectively).
As shown in , pregnancy rates were similar among the three maternal age groups. On the other hand, live birth rates were highest in the 20–30 age group, with a significant decrease with increasing maternal age.
Table 4. Pregnancy and live birth rates by maternal age.
4. Discussion
Controversy remains in literature as to whether male age affects semen quality and male fertility status. Although the relationship between advanced maternal age and declining fertility is well documented, the debate about the impact of age on men continues. The conflicting results of previous studies and the lack of a large-scale study focusing on Turkish men prompted us to further investigate the impact of male age on semen quality and fertility for 8046 men attending an infertility center. To our knowledge, this is the largest study in Turkey aiming to investigate age-related semen parameters and fertility. Our findings show a decline in semen quality and live birth associated with aging.
This study involved men aged between 21 and 65 years, who were analyzed in four age groups. Maternal age was limited to under 45 years and demonstrated an increase with paternal age. The men aged between 31–50 years displayed higher sperm concentrations than those below 30 or above 50 years of age. Progressive motility was highest in men between 31–40 years of age. Both grade A and grade B progressively decreased with advanced male age. Overall, we found a significant decrease in sperm concentration and progressive sperm motility in men over 50 years of age. In accordance with these findings, normozoospermia was least common, and asthenozoospermia and oligoasthenozoospermia were most frequent in men over 50 years of age.
Our results on semen parameters are consistent with many of the previously published studies. Various studies suggest a deterioration in semen quality with advanced male age [Citation8, Citation12, Citation13, Citation23–27]. The optimal semen quality, according to the WHO criteria, is between the ages of 30–35 [Citation2, Citation3]. The highest sperm concentration, total motility, semen volume, total sperm count, and progressive motility are observed between the ages of 30 and 39, and morphological abnormalities are lowest in this age group [Citation28]. Lower sperm concentration and poor sperm morphology are observed in men aged over 40 years [Citation8]. Li et al. reported diminished sperm concentration and lower total sperm count for men above the ages of 58 and 42, respectively [Citation13]. Significant deterioration in sperm parameters and an increased demand for ICSI is detected in men over 50 years of age [Citation25]. When investigating the relationship between age and sperm parameters, numerous studies have examined the impact of sperm chromatin integrity, DNA damage and oxidative stress as potential factors contributing to poor sperm parameters. A recent study reported reduced sperm concentrations accompanied by mitochondrial damage and changes in chromatin density, leading to apoptotic cell death [Citation29]. Lower progressive motility and a higher rate of sperm DNA fragmentation are detected with advancing age in men [Citation30, Citation31]. A recent study revealed elevated sperm chromatin damage and a two-fold increase in the DNA fragmentation rate among men above the age of 40 [Citation32].
However, contrary to the aforementioned findings, some studies report that age does not affect sperm parameters. Age-related differences were not detected when sperm parameters were evaluated in 390 men aged between 21 and 50 years [Citation33]. Similarly, there are additional studies reporting no significant changes in sperm parameters as men age [Citation17, Citation18].
One interesting finding in the current study was the high proportion of azoospermic men aged 21–30 years in comparison to those aged 31–50 years. Similarly, Chen et al. reported lower sperm concentrations in men younger than 25 years compared to those aged 30–45 years [Citation34]. Considering that the best semen quality, according to the WHO criteria, is observed between 30 and 35 years of age [Citation2, Citation3], our findings indicate that sperm production enhances after 30 years of age and declines after 50 years.
The relation of paternal age to the ICSI outcome was another question we sought to answer in the current study. The relationship between male age and ART results is also highly controversial in literature. In the current study, no significant difference was observed when pregnancy rates were compared by paternal age. However, live birth rates demonstrated a significant difference.
Advanced paternal age is significantly associated with elevated risk of miscarriage in ART cycles [Citation14, Citation25, Citation35]. The observed decline in live birth rates in our study may be attributed to the increased risk of spontaneous miscarriage. The highest live birth rates were observed in the 31–40 age group. This group also demonstrated the highest sperm concentration and progressive motility, which were not surprising. Similar to our findings, Lai et al. also reported a deterioration in semen parameters and decrease in live births with increasing age [Citation36]. Negative correlation is observed between male age and live birth rates after ICSI cycles [Citation37]. Clinical pregnancy and live birth rates decrease in men over the age of 50 undergoing ICSI [Citation23]. Advanced paternal age affects ICSI outcomes with donated healthy young oocytes [Citation16].
There are some studies suggesting that increase in paternal age does not affect pregnancy and live birth rates in ART [Citation31, Citation38]. Halvaei et al. proposed that the male age effects pregnancy rates, yet this effect may be due to the fact that most men with advanced age tend to have older female partners [Citation39]. In this study, a dramatic decline in live birth rates was observed in men aged 41–50 years compared to those aged 31–40 years. The mean age of women was similar between these two age groups, indicating the factors other than maternal age alone could influence reproductive outcomes in relation to paternal age.
Most research data indicate that delayed natural conception is more likely to occur with advanced male age. The current study demonstrated a negative influence on pregnancy outcomes with increasing paternal age, which may be due to the increase in embryo aneuploidy rates. García-Ferreyra et al. reported increased DNA damage, reduced blastocyst development rates, and higher rates of aneuploidy for embryos post-ICSI in men aged over 50 [Citation40]. Increasing paternal age is associated with increased male infertility, epigenetic changes, DNA mutations, and chromosomal aneuploidies [Citation41]. Advanced paternal age is linked with reduced natural and intrauterine insemination pregnancy and increased spontaneous miscarriage rates regardless of maternal age [Citation31, Citation42].
The contradictory outcomes reported in the literature could result from methodological discrepancies and variances in the characteristics of study populations. While the concentration of sperm might be affected by both intra- and inter-technician variabilities, the season of sample collection, and statistical methods, sperm characteristics may also be influenced by confounding variables related to the population such as geography, ethnicity, and environmental conditions [Citation43].
2463 cases, either out-of-town or from other hospitals were lost to follow up for pregnancy outcomes, which presents a limitation in the current study. The major strength of this study is its relatively large sample size. To our knowledge, this is the largest study on the effect of age on sperm quality and male fertility in Turkish men undergoing ICSI. This study revealed that advanced male age has unfavorable effects on sperm concentration, progressive motility, and live births.
Authors’ contributions
Study conception and design: KV, HB, MC.
Acquisition of data: KV, HB, YDC, BS, MC.
Analysis and interpretation of data: KV, HB, YDC, BS, MC.
Drafting of manuscript: KV, HB, YDC, BS.
Critical revision: spelling KV, HB, YDC, BS, MC.
All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All participants included in this study were informed and their written informed consent was obtained both for participation and publication. The study protocol was approved by the Non-Interventional Ethics Committee of Maltepe University (approval date: 27.04.2023, approval number: 2023/09-19).
Acknowledgements
The authors would like to thank the staff at Acibadem Hospital’s IVF center for their research support and data screening.
Disclosure statement
No potential competing interest was reported by the authors.
Data availability statement
The datasets analyzed in the present study include all data of a IVF center. Due to the potential for further analysis of the data, it cannot be made publicly available. However, it may be obtained from the corresponding authors upon request.
Additional information
Funding
References
- Eisenberg ML, Meldrum D. Effects of age on fertility and sexual function. Fertil Steril. 2017;107(2):301–304. doi: 10.1016/j.fertnstert.2016.12.018.
- Gunes S, Hekim GNT, Arslan MA, et al. Effects of aging on the male reproductive system. J Assist Reprod Genet. 2016;33(4):441–454. doi: 10.1007/s10815-016-0663-y.
- Levitas E, Lunenfeld E, Weisz N, et al. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia. 2007;39(2):45–50. doi: 10.1111/j.1439-0272.2007.00761.x.
- Zhang Y, Zhang H, Zhao J, et al. Gravidity modifies the associations of age and spousal age difference with couple’s fecundability: a large cohort study from China. Hum Reprod. 2024;39(1):201–208. doi: 10.1093/humrep/dead209.
- Crawford NM, Steiner AZ. Age-related infertility. Obstet Gynecol Clin North Am. 2015;42(1):15–25. doi: 10.1016/j.ogc.2014.09.005.
- Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible? Fertil Steril. 2013;99(1):30–36. doi: 10.1016/j.fertnstert.2012.11.024.
- Kleshchev M, Osadchuk L, Osadchuk A. Age-related changes in sperm morphology and analysis of multiple sperm defects. Front Biosci (Schol Ed). 2023;15(3):12. doi: 10.31083/j.fbs1503012.
- Stone BA, Alex A, Werlin LB, et al. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100(4):952–958. doi: 10.1016/j.fertnstert.2013.05.046.
- Kesari KK, Agarwal A, Henkel R. Radiations and male fertility. Reprod Biol Endocrinol. 2018;16(1):118. doi: 10.1186/s12958-018-0431-1.
- Țarcă V, Țarcă E, Luca FA. The impact of the main negative socio-economic factors on female fertility. Healthcare. 2022;10(4):734. doi: 10.3390/healthcare10040734.
- Danis RB, Samplaski MK. Sperm morphology: history, challenges, and impact on natural and assisted fertility. Curr Urol Rep. 2019;20(8):43. doi: 10.1007/s11934-019-0911-7.
- Crosnoe LE, Kim ED. Impact of age on male fertility. Curr Opin Obstet Gynecol. 2013;25(3):181–185. doi: 10.1097/GCO.0b013e32836024cb.
- Li WN, Jia MM, Peng YQ, et al. Semen quality pattern and age threshold: a retrospective cross-sectional study of 71,623 infertile men in China, between 2011 and 2017. Reprod Biol Endocrinol. 2019;17(1):107. doi: 10.1186/s12958-019-0551-2.
- Kaltsas A, Zikopoulos A, Vrachnis D, et al. Advanced paternal age in focus: unraveling its influence on assisted reproductive technology outcomes. J Clin Med. 2024;13(10):2731. doi: 10.3390/jcm13102731.
- Jimbo M, Kunisaki J, Ghaed M, et al. Fertility in the aging male: a systematic review. Fertil Steril. 2022;118(6):1022–1034. doi: 10.1016/j.fertnstert.2022.10.035.
- Cito G, Coccia ME, Picone R, et al. Impact of advanced paternal age on the intracytoplasmic sperm injection (ICSI) outcomes in donor egg cycles. Transl Androl Urol. 2019;8(Suppl 1):S22–S30. doi: 10.21037/tau.2018.12.13.
- Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95(1):1–8. doi: 10.1016/j.fertnstert.2010.08.029.
- Nijs M, De Jonge C, Cox A, et al. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology: male age, sperm quality, sperm chromatin anomalies and ART outcome. Andrologia. 2011;43(3):174–179. doi: 10.1111/j.1439-0272.2010.01040.x.
- Alviggi C, Conforti A, Carbone IF, et al. Influence of cryopreservation on perinatal outcome after blastocyst‐ vs cleavage‐stage embryo transfer: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;51(1):54–63. Jan; doi: 10.1002/uog.18942.
- Coutifaris C. Freeze-only in vitro fertilization cycles for all? Fertil Steril. 2017;108(2):233–234. doi: 10.1016/j.fertnstert.2017.06.028.
- Gullo G, Basile G, Cucinella G, et al. Fresh vs. frozen embryo transfer in assisted reproductive techniques: a single center retrospective cohort study and ethical-legal implications. Eur Rev Med Pharmacol Sci. 2023;27(14):6809–6823.
- Mevzuat Bilgi Sistemi. [Internet]. [cited 2022 Dec 16]. Available from: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=85319&MevzuatTur=3&MevzuatTertip=5.
- Almeida S, Rato L, Sousa M, et al. Fertility and sperm quality in the aging male. Curr Pharm Des. 2017;23(30):4429–4437.
- Lahimer M, Montjean D, Cabry R, et al. Paternal age matters: association with sperm criteria’s-spermatozoa DNA integrity and methylation profile. JCM. 2023;12(15):4928. doi: 10.3390/jcm12154928.
- Morris G, Mavrelos D, Odia R, et al. Paternal age over 50 years decreases assisted reproductive technology (ART) success: a single UK center retrospective analysis. Acta Obstet Gynecol Scand. 2021;100(10):1858–1867. doi: 10.1111/aogs.14221.
- Nsaif Ali I. Impact of selected lifestyle factors on semen quality in Iraqi men. IJRMST. 2023;16(01):01–10. doi: 10.37648/ijrmst.v16i01.001.
- Verón GL, Tissera AD, Bello R, et al. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil Steril. 2018;110(1):68–75.e4. doi: 10.1016/j.fertnstert.2018.03.016.
- Salmon-Divon M, Shrem G, Balayla J, et al. An age-based sperm nomogram: the McGill reference guide. Hum Reprod. 2020;35(10):2213–2225. doi: 10.1093/humrep/deaa196.
- Condorelli RA, La Vignera S, Barbagallo F, et al. Bio-functional sperm parameters: does age matter? Front Endocrinol (Lausanne). 2020;11:558374. doi: 10.3389/fendo.2020.558374.
- Belloc S, Benkhalifa M, Cohen-Bacrie M, et al. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril. 2014;101(6):1588–1593. doi: 10.1016/j.fertnstert.2014.02.006.
- Belloc S, Hazout A, Zini A, et al. How to overcome male infertility after 40: influence of paternal age on fertility. Maturitas. 2014;78(1):22–29. doi: 10.1016/j.maturitas.2014.02.011.
- Rosiak-Gill A, Gill K, Jakubik J, et al. Age-related changes in human sperm DNA integrity. Aging (Albany NY). 2019;11(15):5399–5411. doi: 10.18632/aging.102120.
- Zabihullah M, Kumar T, Jha K, et al. The effect of age on semen quality among male partners of infertile couples: an observational study in a tertiary care center in Eastern India. Cureus. 2023;15(8):e42882. doi: 10.7759/cureus.42882.
- Chen GX, Li HY, Lin YH, et al. The effect of age and abstinence time on semen quality: a retrospective study. Asian J Androl. 2022;24(1):73–77. doi: 10.4103/aja202165.
- Murugesu S, Kasaven LS, Petrie A, et al. Does advanced paternal age affect outcomes following assisted reproductive technology? A systematic review and meta-analysis. Reprod Biomed Online. 2022;45(2):283–331. doi: 10.1016/j.rbmo.2022.03.031.
- Lai S, Li RH, Yeung WS, et al. Effect of paternal age on semen parameters and live birth rate of in-vitro fertilisation treatment: a retrospective analysis. Hong Kong Med J. 2018;24(5):444–450. doi: 10.12809/hkmj177111.
- Vogiatzi P, Pouliakis A, Sakellariou M, et al. Male age and progressive sperm motility are critical factors affecting embryological and clinical outcomes in oocyte donor ICSI cycles. Reprod Sci. 2022;29(3):883–895. doi: 10.1007/s43032-021-00801-1.
- Tatsumi T, Ishida E, Tatsumi K, et al. Advanced paternal age alone does not adversely affect pregnancy or live‐birth rates or sperm parameters following intrauterine insemination. Reprod Med Biol. 2018;17(4):459–465. doi: 10.1002/rmb2.12222.
- Halvaei I, Litzky J, Esfandiari N. Advanced paternal age: effects on sperm parameters, assisted reproduction outcomes and offspring health. Reprod Biol Endocrinol. 2020;18(1):110. doi: 10.1186/s12958-020-00668-y.
- García-Ferreyra J, Luna D, Villegas L, et al. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health. 2015;9:21–27. doi: 10.4137/CMRH.S32769.
- Sharma R, Agarwal A, Rohra VK, et al. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13(1):35. doi: 10.1186/s12958-015-0028-x.
- Belloc S, Cohen-Bacrie P, Benkhalifa M, et al. Effect of maternal and paternal age on pregnancy and miscarriage rates after intrauterine insemination. Reprod Biomed Online. 2008;17(3):392–397. doi: 10.1016/s1472-6483(10)60223-4.
- Jouannet P, Wang C, Eustache F, et al. Semen quality and male reproductive health: the controversy about human sperm concentration decline note. APMIS. 2001;109(5):333–344. doi: 10.1034/j.1600-0463.2001.090502.x.
