Abstract
Objective: To evaluate the cost-effectiveness of neurothrombectomy with a stent retriever (SolitaireFootnote* Revascularization Device) in treating acute ischemic stroke patients from the UK healthcare provider perspective.
Methods: A Markov model was developed to simulate health outcomes and costs of two therapies over a lifetime time horizon: stent-retriever thrombectomy in combination with intravenous tissue-type plasminogen activator (IV t-PA), and IV t-PA alone. The model incorporated an acute phase (0–90 days) and a rest of life phase (90+ days). Health states were defined by the modified Rankin Scale score. During the rest of life phase, patients remained in the same health state until a recurrent stroke or death. Clinical effectiveness and safety data were taken from the SWIFT PRIME study. Resource use and health state utilities were informed by published data.
Results: Combined stent-retriever thrombectomy and IV t-PA led to improved quality-of-life and increased life expectancy compared to IV t-PA alone. The higher treatment costs associated with the use of stent-retriever thrombectomy were offset by long-term cost savings due to improved patient health status, leading to overall cost savings of £33 190 per patient and a net benefit of £79 402. Deterministic and probabilistic sensitivity analyses demonstrated that the results were robust to a wide range of parameter inputs.
Limitations: The acute and long-term costs resource use data were taken from a study based on a patient population that was older and may have had additional comorbidities than the SWIFT PRIME population, resulting in costs that may not be representative of the cohort within this model. In addition, the estimates may not reflect stroke care today as no current evidence is available; however, the cost estimates were deemed reasonable by clinical opinion.
Conclusions: Combined stent-retriever neurothrombectomy and IV t-PA is a cost-effective treatment for acute ischemic stroke compared with IV t-PA alone.
Introduction
Stroke is a major cause of morbidity and long-term disability both in the UK and worldwide. In the UK, ∼150,000 strokes occur each year, with an estimate of more than 300,000 people living with stroke-related permanent disabilityCitation1,Citation2. Stroke is also a significant cause of mortality in the UK, accounting for ∼53,000 deaths each year, or 9% of all deathsCitation3. Due to the UK’s aging population and increasing prevalence of obesity, the incidence of stroke is set to increase furtherCitation4–6. The substantial health burden of stroke is matched by a substantial economic burden as it results in sizable treatment costs often associated with extensive hospitalization and long-term ongoing care costs after discharge from hospital due to stroke-related disability. The estimated cost of stroke in England is ∼£8.9 billion per year, of which 50% are direct costs to the National Health Service (NHS)Citation7.
Stroke care in the UK has seen significant reorganization over the last decade, with care being increasingly delivered in specialist units, as they have achieved better recovery from strokeCitation8,Citation9. Currently, intravenous tissue plasminogen activator (IV t-PA) represents the best available pharmacological treatment for acute ischemic stroke in the UK as established by the National Institute for Health Care Excellence (NICE) single technology appraisal (STA); therefore, it is the comparator therapy in the present economic evaluation. Recently, endovascular treatment with stent retrievers has emerged as an important treatment option for acute ischemic stroke patients with large vessel occlusionCitation10–14.
While there are clinical data supporting stent-retriever thrombectomy, limited economic evidence to support the routine use of stent retrievers in current stroke care exists. One recent study on the cost-effectiveness of intra-arterial therapy in the US suggested that intra-arterial treatment was a cost-effective treatment with an estimated incremental cost-effectiveness ratio (ICER) of $14,137 (£10,011) per quality-adjusted life year gained (QALY)Citation15. This analysis was based on data from the MR CLEAN trial, which includes use of various intra-arterial interventions including stent retrievers, thromboaspiration, and intra-arterial thombolysis. The aim of our study was to evaluate from the UK healthcare provider perspective the cost-effectiveness of combined stent-retriever (SolitaireFootnote* Revascularization Device) thrombectomy and IV t-PA compared to IV t-PA alone as a treatment for acute ischemic stroke by leveraging the recently available data from the Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) Clinical TrialCitation10.
Methods
Model overview
The model was designed in collaboration between stroke clinicians and experts in healthcare economics and was based on a pragmatic review of previous decision analytical models developed in stroke care. On consideration of the available data, a model structure similar in design to that used in the NICE single technology appraisal of alteplase was developedCitation16. Clinical efficacy and safety data used in the analysis was sourced from the SWIFT PRIME study—a multi-center randomized clinical trial conducted in the US and Europe on patients suffering from large vessel anterior circulation occlusions (ClinicalTrials.gov Identifier: NCT01657461)Citation10. The study compared stent-retriever thrombectomy in combination with IV t-PA, with IV t-PA alone. A targeted literature search was undertaken to populate the model inputs that were not provided by SWIFT PRIMECitation10 trial data, such as the costs related to acute ischemic stroke and the recurrence of stroke.
This economic analysis was conducted assuming a UK healthcare provider perspective and a lifetime time horizon was considered. Outcomes were also evaluated at after 1, 2 and 5 years. All costs were inflated to 2013/2014 prices using the Hospital and Community Services Inflation IndexCitation17. Health outcomes and future costs were discounted at 3.5% following NICE recommendationsCitation18. The model was developed and run in Microsoft Excel 2010.
Patients
Patients in the model were based on the population in the SWIFT PRIMECitation10 trial. Institutional Review Boards/Ethics Committees at sites approved the trial and all subjects provided informed consent. Briefly, patients were eligible for enrollment into the trial if they had confirmed occlusions in the proximal anterior intracranial circulation and an absence of large ischemic-core lesions. Moreover, patients in the combined stent-retriever and IV t-PA arm had to undergo mechanical thrombectomy within 6 h of symptom onset. For the present analysis, starting age in the base case and scenario analysis was 66, the mean age of the SWIFT PRIMECitation10 trial population. There was no participating center located in the UK.
Model structure
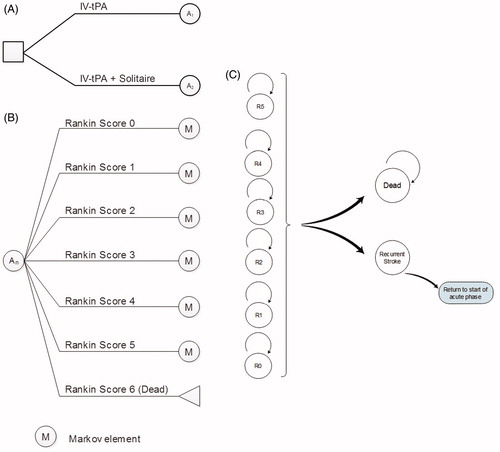
A Markov decision model was used to simulate and predict long-term health outcomes and costs of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone. A diagrammatical depiction of the model structure is presented in . In the base case, the model structure used two distinct phases to model patient outcomes, including an acute phase and a rest of life phase. The acute phase modeled patients from stroke onset to 90 days (), in which they were assumed to receive treatment, and a rest of life phase spanning from 91 days to the end of the patients’ lives. A total of seven health states were used in the model based on the modified Rankin Scale (mRS). The scale ranges from 0–6 and is defined as; mRS 0 = no symptoms, mRS 1 = no clinically significant disability, mRS 2 = slight disability, mRS 3 = moderate disability, mRS 4 = moderately severe disability, mRS 5 = severe disability, and mRS 6 is deadCitation19. Death was the only absorbing state within the model. All treatment effects were assumed to occur within the acute phase and a patient was assigned an mRS score at 90 days and at 7 days to allow for half cycle correction. During the acute phase, the patient was at risk of two different adverse events; symptomatic hemorrhage and vasospasm. Experiencing an adverse event incurred additional costs; however, no disutility was applied as it was assumed that quality-of-life was reflected in the 90 day mRS. The vasospasms that occurred in SWIFT PRIME were intra-procedural and were brief and asymptomatic. Because of this, it is unlikely that the vasospasms in SWIFT PRIME will have resulted in any additional costs; however, a conservative approach was taken and the costs related to the injection to Nimotop were included within the model. For similar reasons, it was also assumed that a patient was at no risk of recurrent stroke in this 90-day period. Effectiveness data and risk of adverse events were populated from the SWIFT PRIMECitation10 trial.
Table 1. Clinical data input—clinical efficacy (acute phase).
In the base case, the second phase of the model was made up of two cycle lengths. The first cycle length was 275 days, bringing a patient’s time after stroke to 1 year. After this, the cycle length became from 1 year onwards. In the rest of life phase of the model, a patient remained in the same mRS as at 90 days until either a recurrent stroke or death. Recurrent stroke probabilities were taken from Mohan et al.Citation20, who used a statistical model to demonstrate time trends in risk of recurrent stroke to predict future trends (). Cumulative risk at 1 year and at 5 years after first stroke were reported and these were used as the risk in the first cycle of the rest of life phase (90 days to 1 year) and the annual cycles after the first year, respectively, within our model. These risks were adjusted to reflect the two different cycle lengths within the model. Risk of recurrent stroke was the same across all mRS scores and it was assumed that a patient could experience a maximum of one recurrent stroke per cycle.
Table 2. Clinical data input—mortality and recurrent stroke.
If a patient experienced a recurrent stroke, the patient re-entered the model from the start with restricted movement. That is, a patient with a recurrent stroke was only able to have an mRS score equal to or greater than their mRS before that recurrent stroke. The patient then continued through the model as previously described.
In a scenario analysis, a further stage was added to the model to create a three stage structure. These three phases were the acute phase from 0–90 days, a rehabilitation phase from 91 days to 1 year, and a rest of life phase from 1 year onwards. In the rehabilitation phase, patients could either remain in the same health state (maintain their 90 day mRS score), improve by one health state, or deteriorate by one health state (). The rehabilitation phase was included based on evidence that a patient’s recovery from stroke is not complete at 90 days. Therefore, patients can often have different mRS scores at 1 year follow-up due to rehabilitation and other factorsCitation21–23. Within the rehabilitation phase, as in the rest of life phase, a patient was at risk of recurrent stroke and could only experience one recurrent stroke in this phase. The rehabilitation phase was not included in the base case due to a lack of reliable data on patients’ likely progression through this phase. It was instead included in a scenario analysis to explore the potential impact of post 90-day improvement in mRS score on estimated cost-effectiveness.
Table 3. Clinical data input—clinical efficacy (rehabilitation phase).
Costs and resource use
Costs and resource use in the model are presented in and . Costs can be broken down into treatment costs, costs in the acute phase, and costs in the rest of life phase. The overall costs included device and drug costs, costs of administering treatment (e.g. staff and overhead costs), management of adverse events, hospitalization costs, and long-term care costs. Treatment and device costs for the stent retriever were provided by Medtronic and the resource use (e.g. staff and theatre time for stent-retriever thrombectomy) was elicited from clinicians with costs sourced from Personal Social Services Research Unit (PSSRUCitation17), Unit Costs of Health & Social Care. Costs and resource use associated with IV t-PA were derived from the Single Technology Appraisal for alteplase (TA122)Citation16,Citation24,Citation25 and PSSRU, and were updated to 2014 figures where appropriateCitation26. Long-term costs were based on data from the OXVASC studyCitation27, and additional nursing and residential care costs, also reported in the study, were included to reflect personal social services costs as required by the NICE reference caseCitation28. Data was only available for three levels of post-stroke disability; mRS 0–2, mRS 3–4, and mRS 5. Based on the consensus of three clinical experts, a weighting on the three levels was applied in order to calculate individual costs by mRS. The net monetary benefit was calculated by multiplying the incremental total QALYs by the willingness-to-pay threshold of £20,000. The incremental total costs were then subtracted from this. This allows for the additional QALYs to be valued in monetary terms and the overall monetary benefit estimated by taking into account the extra costs/cost-savings associated with the intervention.
Table 4. Treatment costs of stent retriever and IV-tPA.
Table 5. Acute and long-term costs of acute ischemic stroke by mRS.
Quality-of-life
Utilities were assigned to each health state and were based on patients in the Oxford Vascular Study (OXVASC), UKCitation29. Utility values for the health states ranged from 0.935 to −0.054, where an mRS score of 5 resulted in a negative utility, indicating that living with mRS 5 is worse than death. presents the health state utility values used in the model.
Table 6. Utility data inputs.
Mortality
Age-specific other-cause mortality was applied to patients in the rest of life phase of the model (1 year+) to model deaths unrelated to stroke. Mortality data from the 2011–2013 Office of National Statistics Life Tables were usedCitation30. Relative risks of dying by mRS (values reported in ) were also applied to other-cause mortality in this phase and were taken from Slot et al.Citation31. It was assumed that mRS 0 could be taken as baseline, and that a patient with mRS 0 had the same risk of dying as anyone within the same demographic that had not experienced a stroke.
Model outcomes
A number of economic outcomes were calculated as part of the analysis. The primary outcome of the analysis is the incremental cost-effectiveness ratio (ICER). The ICER was calculated by dividing the incremental costs by the incremental QALYs. Net monetary benefit (NMB) was calculated using a £20,000 threshold, using recommendations of the NICE reference case (NMB = (ΔQALYs × λ) − ΔCosts, where ΔQALYs is the difference in QALYs, ΔCosts is the difference in costs, and λ is the threshold value adopted)Citation28. Costs and QALYs were evaluated over the patient lifetime, and at 1, 2, and 5 years. In addition to these generic measures of cost-effectiveness, a number of disease-specific measures of cost-effectiveness were calculated, specifically the average cost per mRS score avoided, the cost per additional independent patient (mRS <3) and the cost saved (by mRS) over a patient’s lifetime due to improvement by one mRS.
A number of deterministic sensitivity analyses were performed to explore uncertainty around input parameter values. Probabilistic sensitivity analysis was also undertaken to assess overall uncertainty in the model. This was implemented by assigning a probability distribution to key parameters to represent the uncertainty around its mean value; one value was sampled from each distribution and the results calculated using these sampled inputs. This process was repeated 1000 times to generate repeated estimates of the costs and QALYs associated with each intervention. mRS at 90 days was modeled using a Dirichlet distributionCitation32. Cost inputs were varied according to a gamma distribution, and relative risks of mortality were varied according to a log-normal distribution. Utilities were varied according to a beta distribution, with alpha and beta estimated from the mean and SE values. The internal validity of the model was checked by a health economist of York Health Economist Consortium not involved in the development of the model.
Results
The model predicted that the use of stent-retriever thrombectomy in combination with IV t-PA for the treatment of acute ischemic stroke was associated with improved patient outcomes and lower overall costs compared with IV t-PA alone over the patient’s lifetime (). The higher cost associated with the stent retriever was offset by the cost savings resulting from lower long-term care costs due to improved patient outcomes, with an overall cost saving of £33,190 per case. Higher QALYs and life years were observed in the stent retriever + IV t-PA arm (a difference of 2.31 and 1.81, respectively), reflecting the higher mortality rate and lower quality-of-life in patients with more severe stroke outcomes. As stent retriever + IV t-PA led to greater health gains at a lower cost; it was dominant compared with IV t-PA alone. The net monetary benefit (NMB) per case was £79,402 per QALY.
Table 7. Base case results of the cost-effectiveness of Stent retriever + IV t-PA vs IV t-PA alone (discounted lifetime costs and QALYs per patient).
At 1 year, stent retriever + IV t-PA was demonstrated as being approximately cost-neutral, with increased costs of £62 per patient compared with IV t-PA alone. At 2 years, stent retriever + IV t-PA was associated with overall cost savings.
As stent retriever + IV t-PA was cost-saving, both the average cost per mRS score avoided at 90 days and per additional independent patient at 90 days were negative values (cost savings); −£33,486 and −£132,064, respectively. The costs saved due to improvement by one mRS varied from £5248 ($7411) (moving from mRS 2 to mRS 1) and £111,209 (moving from mRS 3 to mRS 2). The cost savings associated with stent retriever + IV t-PA were greatest in patients with higher functional dependence (mRS 3+). The full breakdown by mRS can be found in .
Analysis of the components of the overall cost showed major differences in the long-term care costs between treatment arms. The largest cost savings were observed post-discharge, since stent retriever + IV t-PA was demonstrated to reduce the risk of long-term disability compared with IV t-PA alone. The lifetime long-term care cost was £86,137 for patients who had received stent retriever + IV t-PA, compared with £123,057 for patients who had received IV t-PA alone.
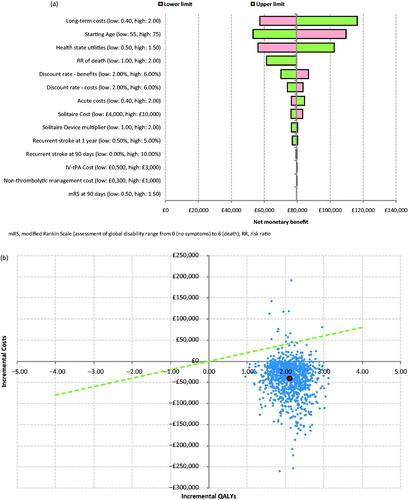
Deterministic sensitivity analysis indicated that stent retriever + IV t-PA remained a cost-effective treatment option across a large range of parameter values. The starting age, health state utilities and long-term costs were the key drivers of the analysis, as indicated by the tornado diagram in . A higher starting age, lower long-term costs, and lower health state utilities were associated with a lower net benefit.
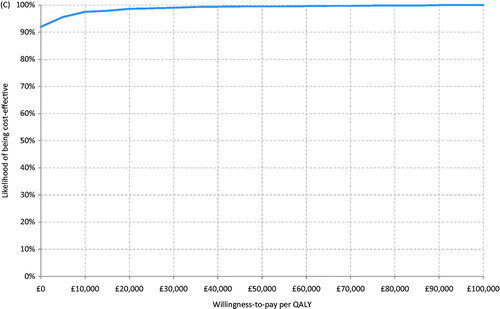
Figure 2. (a) Tornado diagram showing results of deterministic sensitivity analysis. (b) Probabilistic sensitivity analysis—scatter plot. (c) Probabilistic sensitivity analysis—CEAC.
A scenario analysis was undertaken, whereby it was assumed that patients entered a rehabilitation phase after the acute phase, lasting from 90 days to 1 year, where they were able to improve or deteriorate by one health state. In this scenario, stent retriever + IV t-PA remained a dominant treatment option and was associated with cost savings of £28,134 and a net benefit of £72,883, which, while still favorable for stent-retriever thrombectomy, is less cost-effective than in the base case analysis. Both treatment arms were associated with higher QALYs and lower long-term costs than the base case analysis. A scenario where long-term costs included in the model consisted only of hospital costs (i.e. nursing and residential costs excluded) resulted in lower overall costs (cost saving of £894) and a lower net benefit of £47,106.
Probabilistic sensitivity analysis indicated that the use of stent retriever + IV t-PA had a 98.6% likelihood of cost-effectiveness compared with IV t-PA alone for a £20,000 per QALY threshold value, and a 99.0% likelihood of cost-effectiveness for a £30,000 threshold value.
Discussion
The objective of this analysis was to leverage clinical data specifically from the SWIFT PRIME trial to estimate the cost-effectiveness of stent-retriever thrombectomy in the SWIFT PRIME trial. The model predicted that the stent-retriever thrombectomy in combination with IV t-PA for the treatment of patients with acute ischemic stroke due to proximal anterior intracranial circulation occlusion was cost-saving, with overall savings of £33,190 per treated patient when compared with IV t-PA alone. The NMB was £79,402 per QALY. These results were robust to a large range of parameters in the deterministic sensitivity analyses, and treatment with a stent retriever in combination with IV t-PA had a 98.6% probability of being cost-effective at a threshold value of £20,000 per QALY in the probabilistic sensitivity analyses.
A main strength of the study was that the efficacy and adverse event data was based on Phase III trial data from SWIFT PRIMECitation10—a randomized, open label, blinded outcome observer trial that was stopped early due to strong evidence of efficacy combined stent-retriever thrombectomy and IV t-PA. An additional strength was the inclusion of recurrent stroke in the analysis to ensure that all clinical outcomes following a stroke were modeled as well as the costs associated with them. Further, the model links health status by mRS to patient outcomes of mortality, quality-of-life and resource use. Individual mRS scores, as opposed to grouped health states of independent, dependent, and dead, were also used for a clearer breakdown on the patients’ pathway.
We used key clinical data from the SWIFT PRIME study to evaluate the short-term costs and health outcomes. However, the implications of stroke (in terms of cost, quality-of-life, and mortality) must be evaluated in the long-term to fully capture the relative benefits of competing interventions. We therefore supplemented this approach with data from alternative studies to predict costs and outcomes over a lifetime horizon, and, although the absence of long-term clinical data from SWIFT PRIME is a limitation of the analysis, the results suggest that the model is robust to changes in the input parameters.
To date, only one other study has been conducted on the cost-effectiveness of the use of stent retrievers in combination with IV t-PA therapy compared with IV t-PA alone. In this studyCitation15, an analysis in a US setting, intra-arterial therapy was associated with an ICER of $14,137 (£10,011) and was estimated to have a probability of cost-effectiveness of 97.6% at a threshold of $50,000 (£35,407). Using a £20,000 threshold, as used in this analysis, the intervention would have a lower probability of cost-effectiveness. The Leppert study was based on clinical efficacy data from MR CLEANCitation11, a clinical trial that utilized a variety of endovascular therapies for acute ischemic stroke, including intra-arterial instillation of fibrinolytic drugs, use of thromboaspiraton devices, coil retrievers, and stent retrievers that had received a Conformité Européenne (CE) marking. Intra-arterial treatment in this study was associated with lower clinical effectiveness than that observed in the SWIFT PRIMECitation10 study, which focused exclusively on stent retrievers. The absolute between-group difference in the proportion of patients who were functionally independent (mRS 0–2) at 90 days in MR CLEAN was 13.5 percentage points in favor of the intervention group (32.6% vs 19.1%), while in SWIFT PRIME the proportion of patients who were functionally independent at 90 days was 25 percentage points in favor of the intervention group (60% vs 35%). Mortality at 90 days did not differ significantly between groups in both trials; however, the rate observed in the intervention group was higher in MR CLEAN compared to SWIFT PRIME, 21% vs 9%, respectively. In addition to the different mechanical thrombectomy devices utilized in these two trials, other factors such as differences in target patient populations, differences in time to reperfusion and reperfusion rates achieved in the treatment arms of both studies may have influenced the clinical effectiveness of mechanical thrombectomy. Imaging criteria was less stringent in MR CLEAN, requiring evidence of intracranial arterial occlusion only for inclusion of patients, while SWIFT PRIME also required evaluation of ischemic core size/salvageable area via perfusion imaging/ASPECTS. Eighty-seven per cent of patients in the interventional arm of MR CLEAN received IV t-PA, whereas SWIFT PRIME included only patients initially treated with IV t-PA. More importantly, successful reperfusion (Thrombolysis in Cerebral Infarction (TICI) 2b-3 grade flow) was achieved in 88% and 58.7% of patients in the intervention groups in SWIFT PRIME and MR CLEAN, respectively. Time from stroke onset to reperfusion was also longer in the MR CLEAN cohort vs SWIFT PRIME cohort. Lastly, these studies were carried out at different centers and thrombectomies were performed by different surgeons. Collectively, these differences discussed above appear to have translated into differences in clinical outcomes achieved across the studies. In addition, there was a significant difference between the two studies in the utility values used. The utilities in the Leppert study were derived from Samsa et al.Citation33—a study that assessed patient preferences for hypothetical major stroke using a patient survey, whilst utilities used in this study were derived from patients with stroke identified as part of the Oxford Vascular study (OXVASC)Citation29. Utilities used in this study were higher than those reported by Samsa et al. for the four health states corresponding to mRS 0 to mRS 3. These differences in utilities are likely to have led to improved cost-effectiveness within our study, as the additional gains in quality-of-life in the less severe health states will lead to improved QALY gains when patients move from mRS levels of 4 or higher to mRS levels of 1–3. Another key difference between the two cost-effectiveness studies is in the acute and long-term costs used. The costs used in the Leppert study were sourced from Earnshaw et al.Citation34 and Chambers et al.Citation35, and are reflective of the US healthcare system. The costs were significantly lower in the Leppert study, which will have contributed to the lower cost-effectiveness in that study.
A cost-effectiveness analysis of alteplase in the treatment of acute ischemic stroke within 4.5 h of symptom onset was undertaken for a NICE Single Technology Appraisal in 2012, taking a UK healthcare perspectiveCitation16. In this analysis, alteplase was found to have an ICER of £2441 per QALY when compared to no treatment (placebo). The life-time QALYs gained by alteplase were 3.307 per patient, and the lifetime cost of alteplase was £29,330 per patient. Comparing these results to our analysis, stent-retriever thrombectomy in combination with IV t-PA was dominant over a lifetime time horizon when compared to IV t-PA alone. The lifetime QALYs gained by using the stent retriever were 7.01, and the lifetime costs associated with the stent retriever were £141,564. In addition to this, the lifetime cost of IV t-PA within our analysis was £183,897. This cost was substantially higher than that used within the alteplase STA. This is likely to be a result of higher health-state cost inputs in our analysis and a higher mortality observed within the alteplase model. Resource use costs used within our analysis are more recent, dating from 2008–2009, compared to costs from 2001 in the alteplase analysis. Because of this, the costs used within this analysis may be more representative of the current patient pathway.
There are potential limitations of this study in relation to the resource use data used. Both acute and long-term costs were taken from a study by Luengo-Fernandez et al.Citation27, which is based on patients with atrial fibrillation with an average age of 80 years, followed between 2002–2007. As the patients are older than the SWIFT PRIMECitation10 population, and also suffer from atrial fibrillation, there may be additional comorbidities in this population, and the costs may not be representative of the cohort within our model. The estimates may also not be reflective of stroke care today. However, no other evidence is currently available, and the costs were verified to be reasonable estimates by clinical opinion. Further research into the costs of long-term stroke care and the benefits of long-term rehabilitation may be warranted as it would have significantly reduced the uncertainty around estimated cost-effectiveness estimates in the current model.
The economic aspect of this analysis focused only on the costs incurred to the NHS; a societal perspective, incorporating the wider economic impact of improving stroke outcomes (for example, by including the possibility of patients and their families returning to work) would most likely improve the cost-effectiveness profile of the stent retriever still further. One study concluded that the treatment of and productivity loss arising from stroke results in total societal costs of £8.9 billion a year, with treatment costs accounting for ∼5% of total UK NHS costsCitation7.
Conclusion
Combined stent-retriever neurothrombectomy and IV t-PA is a highly effective treatment for acute ischemic stroke and results in long-term cost-saving when compared with IV t-PA alone. These findings establish a strong case for introducing stent-retriever thrombectomy into standard stroke care.
Transparency
Declaration of funding
This work was funded by Medtronic, the manufacturer of Solitaire.
Declaration of financial/other relationships
KL has received consulting and speakers honoraria from Medtronic. RV has received consulting and speakers honoraria from Bayer, BMS Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Medtronic, Morphosys, as well as research support from Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo. IHC, LMC, and RH are employed by York Health Economics Consortium. York Health Economics Consortium received funding from Medtronic for conducting health economics research and consultancy. JLS is an employee of the University of California. The University of California, Regents receive funding for JLS’ services as a scientific consultant regarding trial design and conduct to Medtronic/Covidien, Stryker, Neuravi, BrainsGate, Pfizer, Squibb, Boehringer Ingelheim (prevention only), ZZ Biotech, and St. Jude Medical. JLS serves as an unpaid consultant to Genentech, advising on the design and conduct of the PRISMS trial; neither the University of California nor JLS received any payments for this voluntary service. The University of California has patent rights in retrieval devices for stroke.
Acknowledgment
This data has been presented at the 13th Congress of the World Federation of Interventional and Therapeutic Neuroradiology, 2015.
Notes
*Solitaire Revascularization Device is a registered trademark of Medtronic (Irvine, CA).
*Solitaire Revascularization Device is a registered trademark of Medtronic (Irvine, CA).
References
- Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773–83
- Wolfe C, Rudd A. The burden of stroke white paper. London, UK: The Stroke Association, 2007
- British Heart Foundation, University of Oxford. British Heart Foundation Health Promotion Research Group. Stroke Statistics 2009. London: British Heart Foundation, 2010
- O'Donnell MJ, Xavier D, Liu LS, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112-23
- Dunnell K. Ageing and mortality in the UK—national statistician’s annual article on the population. Popul Trends Winter 2008;134:6-23
- Johnson W, Li L, Kuh D, et al. How has the age-related process of overweight or obesity development changed over time? Co-ordinated analyses of individual participant data from five United Kingdom birth cohorts. PLoS Med 2015;12:e1001828
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009;38:27-32
- Bray BD, Ayis S, Campbell J, et al. Associations between the organisation of stroke services, process of care, and mortality in England: prospective cohort study. BMJ 2013;346:f2827
- Morris S, Hunter RM, Ramsay AI, et al. Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014;349:g4757
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306
- Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-18
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30
- Leppert MH, Campbell JD, Simpson JR, et al. Cost-effectiveness of intra-arterial treatment as an adjunct to intravenous tissue-type plasminogen activator for acute ischemic stroke. Stroke 2015;46:1870-6
- Boehringer Ingelheim. Alteplase for treating acute ischaemic stroke - Single Technology Appraisal; Manufacturer's Submission. 2012
- PSSRU. Unit Costs of Health & Social Care 2014. Canterbury: University of Kent, 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014/. Accessed 22 June 2015
- HM Treasury. The Green Book - Appraisal and Evaluation in Central Government. London, UK: 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/220541/green_book_complete.pdf. Accessed 22 June 2015
- Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7
- Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489-94
- Jung S, Mono ML, Fischer U, et al. Three-month and long-term outcomes and their predictors in acute basilar artery occlusion treated with intra-arterial thrombolysis. Stroke 2011;42:1946-51
- Luengo-Fernandez R, Paul NL, Gray AM, et al. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013;44:2854-61
- Nedeltchev K, Fischer U, Arnold M, et al. Long-term effect of intra-arterial thrombolysis in stroke. Stroke 2006;37:3002-7
- ISD Scotland. Costs Book tables: R130 expenditure and activity - laboratory services. Edinburgh, Scotland: ISD Scotland, 2014. http://www.isdscotland.org/Health-Topics/Finance/Costs/File-Listings-2014.asp#1338. Accessed 22 June 2015
- Joint Formulary Committee. British National Formulary (online). London, UK, 2015. http://www.medicinescomplete.com. Accessed 22 June 2015
- Department of Health. NHS Reference Costs 2014. London, UK, 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014. Accessed 22 June 2015
- Luengo-Fernandez R, Yiin GS, Gray AM, et al. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke 2013;8:308-14
- National Institute for Health and Care Excellence (NICE). Process and methods guides - Guide to the methods of technology appraisal 2013. London, UK: National Institute for Health and Care Excellence (NICE), 2013. http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf. Accessed 22 June 2015
- Rivero-Arias O, Ouellet M, Gray A, et al. Mapping the modified Rankin scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54
- Office for National Statistics. Life expectancy at birth and at age 65 by local areas in England and Wales: 2011–2013. London, UK: Office for National Statistics, 2014. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/lifeexpectancyatbirthandatage65bylocalareasinenglandandwales/2014-11-19. Accessed 22 June 2015
- Slot KB, Berge E, Sandercock P, et al. Causes of death by level of dependency at 6 months after ischemic stroke in 3 large cohorts. Stroke 2009;40:1585-9
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: OUP, 2006
- Samsa GP, Reutter RA, Parmigiani G, et al. Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol 1999;52:259-71
- Earnshaw SR, Wilson M, Mauskopf J, et al. Model-based cost-effectiveness analyses for the treatment of acute stroke events: a review and summary of challenges. Value Health 2009;12:507-20
- Chambers MG, Koch P, Hutton J. Development of a decision-analytic model of stroke care in the United States and Europe. Value Health 2002;5:82-97