Abstract
Objective: Propel is a bioabsorbable drug-eluting sinus implant inserted following an endoscopic sinus surgery (ESS) for chronic rhinosinusitis (CRS). The objective of this study was to estimate the budget impact of incorporating Propel post-ESS for CRS patients from a self-insured employer or third-party payer perspective.
Methods: An Excel-based budget impact model was developed. Estimates of the prevalence of CRS, rates of ESS, and effectiveness outcomes, along with direct and indirect costs from CRS were obtained from published literature. A total population of 1.5 million members was hypothesized for the analysis. All cost data were adjusted to October 2015 US dollars using the Medical Care Component of the Consumer Price Index. The cost and clinical/economic characteristics of Propel were compared to other treatments commonly used to minimize post-operative complications. The primary outcome was the incremental budget impact reported using per-member-per-month (PMPM) costs. Scenario-based, probabilistic, and one-way sensitivity analyses were performed to gauge the robustness of the results and identify the parameters with the most influence on the results.
Results: For a US self-insured employer or a commercial health plan of 1.5 million members, the incremental PMPM impact of incorporating Propel was estimated to range from −$0.003 to $0.036, respectively, for all members in the health plan. Sensitivity analyses identified the cost of Propel, probability of polyposis recurrence requiring medical intervention, probability of adhesion formation requiring surgical intervention, and the treatment costs for polyposis as the primary parameters influencing the results.
Conclusion: This study has demonstrated the use of Propel following ESS procedures has a negligible impact on the budget of a US self-insured employer or payer. The upfront cost of Propel was offset by savings associated with reduced probability for polyp recurrence, adhesion formation, and their subsequent treatment.
Background
Chronic rhinosinusitis (CRS) affects an estimated 2–16% of the populationCitation1–3 and produces an estimated $9.9–$12.5 billion in annual direct healthcare costsCitation1,Citation4, with an additional $13.1 billion attributable to indirect costsCitation4. Given the economic impact of CRS on self-insured employers, third party payers, and society, it is important to critically evaluate the interventions to ensure clinicians are providing high-value care. Primary CRS symptom management involves a broad spectrum of medical therapies including topical/oral corticosteroids, saline irrigations, oral antibiotics, and leukotriene receptor antagonistsCitation5. Due to the multifactorial etiology of CRS involving both host and environmental factorsCitation6, a successful management strategy often requires multiple concurrent medical therapies. However, despite appropriate medical management, medical therapy alone fails to control sinonasal symptoms in an estimated 60% of cases, and ESS is offered as an adjunctive treatment optionCitation7.
Appropriate post-operative care is an important component to help ensure successful outcomes following endoscopic sinus surgery (ESS). Evidence indicates that, if persistent inflammation is adequately treated following surgery, the chance of revision surgical intervention is essentially eliminated over a nearly 8-year follow-up periodCitation8. Subsequently, a combination of post-operative care strategies is recommended to optimize wound healing and minimize mucosal inflammationCitation9. Evidence-based strategies include high-volume saline irrigations, topical corticosteroid sprays, and a sinus cavity debridement. However, during the early post-operative period, the sinus cavity is often obstructed by crusting and retained secretions which limit the access and effectiveness of topical corticosteroid therapy. To overcome this limitation, steroid-eluting implants have been developed and inserted into the sinus cavities after ESS.
The Propel (Intersect ENT, Palo Alto, CA) bioabsorbable drug-eluting sinus implant (BDESI) was approved by the US Food and Drug Administration (FDA) in 2011. Propel is made of a biodegradable polymer with a non-obstructive design that is placed into the dissected ethmoid sinus cavity. It self-expands upon contact with the mucosa, gradually eluting 370 μg of mometasone furoate over a period of ∼30 days before dissolving in 30–45 days. A meta-analysis of two randomized clinical trials (RCTs) demonstrated that Propel improved ESS outcomes by reducing post-operative scarring, inflammation, polyposis, and middle turbinate lateralizationCitation10. By maintaining patency, it is expected to reduce the need for post-operative interventions such as surgical adhesion lysis and/or use of oral corticosteroids. Rudmik and SmithCitation11 also found that Propel may be a cost-effective option vs non-drug eluting sinus implant for preventing a clinical intervention within 60 days following ESS.
While establishing cost-effectiveness of a new intervention is vital, in the current environment of escalating healthcare expenditures, it is also important to understand if adopting a new technology is affordable. In light of Propel’s promising clinical results, policy-makers and third party payers must consider the financial impact of introducing Propel into the current post-ESS care pathway. This study seeks to provide such evidence by evaluating the incremental budgetary impact of incorporating a BDESI (Propel) for refractory CRS patients undergoing ESS from the perspective of a US commercial payer and self-insured employer.
Methods
An MS Excel-based budget impact model was developedCitation12 and validated using a decision tree simulation model (TreeAge Pro 2015 analysis software) following ISPOR’s Good Practice Report guidelinesCitation13. The impact of incorporating Propel on the budget of a US self-insured employer or commercial third party payer was assessed for a hypothetical population of 1.5 million adult members within a 6-month time horizon. The primary outcome was the incremental budget impact of Propel reported in terms of per-member-per-month (PMPM) costs across the entire health plan. The model was designed to simulate the clinical management of a refractory CRS patient undergoing ESS and receiving one of two treatment strategies: (1) placement of Propel (intervention) or (2) placement of non-drug eluting bioabsorbable or non-absorbable packing material (non-Propel). The non-Propel group of patients was expected to also receive daily topical corticosteroid nasal sprays and high-volume saline irrigationsCitation14. It was assumed that both patient groups follow the same follow-up care plan in terms of healthcare visits with incremental visits contingent on complication occurrence and/or frequency.
A best-evidence review of the published literature was conducted to inform the model inputs. This approach consisted of a literature search of English language peer-reviewed articles and government-sponsored reports indexed in the National Library of Medicine’s PubMed database (PubMed) and Google Scholar from January 2000 to May 2015. Population-based inputs included prevalence of CRS disease in a general population (2%)Citation3, and rate of ESS; that is, the prevalence of CRS patients undergoing ESS (11.4%)Citation15. Model parameters between the two comparator arms were assessed at 1–2, 3–5, and 6-month post-ESS intervals. (See for the model scaffold, footnotes for additional information.)
Figure 1. Decision tree/model scaffold. Adapted from Brosa et al.Citation12. CRS, chronic rhinosinusitis; ESS, endoscopic sinus surgery.
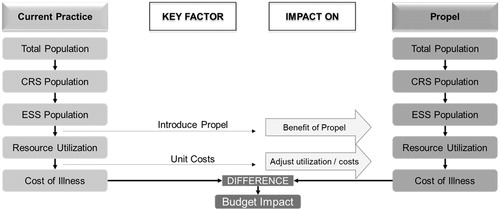
Table 1. Clinical and utilization parameters.
Clinical data
These model inputs included the rate of post-operative complications necessitating a clinical intervention (and therefore medical visit(s)) in addition to the post-operative treatment assumed in the control group and CRS recurrence leading to the revision ESS procedure. The three potential complications of interest included: (1) probability of severe adhesion/synechiae requiring a lysis procedure; (2) probability of recurrent polyposis requiring a 2-week rescue course of oral corticosteroids; and (3) probability of revision sinus surgery. For the purposes of this analysis, we assumed that all CRS patients with symptom recurrence severe enough to warrant a revision surgery recommendation elected to receive it at the end of 6 months.
Pooled results from the Han et al.Citation10 meta-analysis provided parameter values for the Propel arm. For the non-Propel arm, sample-size weighted estimates were used to populate the parameter values for the base-case analysisCitation16–21. The minimum and maximum values obtained from this review were used to inform the sensitivity analyses. For revision surgery rates, we adjusted for both the sample size and the follow-up length of the source studiesCitation22–30. Where the available literature did not yield estimates for the time points of interest (e.g. likelihood of lysis of adhesions in the Propel arm for months 3–5 and 6), parameter values were imputed assuming (a) constant absolute change (ΔPropel-non-Propel), and (b) constant rate of change across the time points for any intervention (see footnotes for additional information).
Cost data
The cost of Propel was calculated after obtaining the average selling price of each implant from Intersect ENT Inc. ($695) and assuming that 80% of ESS patients underwent a bilateral surgery (and therefore needed two implants), while the rest required unilateral surgery (and, hence, one implant)Citation31. The total packing and treatment costs for non-Propel included the sum of the costs of the packing materials, nasal sprays, irrigants, and corticosteroid therapy for the duration of their treatment period.
Treatment for post-operative lysis of adhesion was defined to include a physician visit (CPT# 99214), endoscopy/debridement (CPT# 31231), and in-office lysis procedure (CPT# 30560). Severe polyp recurrence was defined to include a physician visit (CPT# 99214), endoscopy/debridement (CPT# 31231), and a 2 week course of 30 mg of prednisone (oral corticosteroid). Direct healthcare costs for the relevant diagnostic tests, procedures, physician visits, and treatments were obtained from a recent studyCitation11. Because that study employed Medicare costs, these were adjusted by 145% (range =135–155%) to represent costs from a commercial payer perspectiveCitation32. All unit costs were then inflated to October 2015 US dollars using the Medical Care Component of the Consumer Price IndexCitation33. Average wholesale prices were used as the source of prescription drug prices for nasal corticosteroidsCitation34. For the indirect cost burden, it was posited that each office visit would include any necessary labs and/or clinic visits and would involve 4 hours, including travel time. To estimate the value of lost work, the total number of missed days was summed and multiplied by the 2015 average daily wage estimated at $201.60 (Bureau of Labor Statistics)Citation35. All model parameters and values are summarized in .
Sensitivity analyses
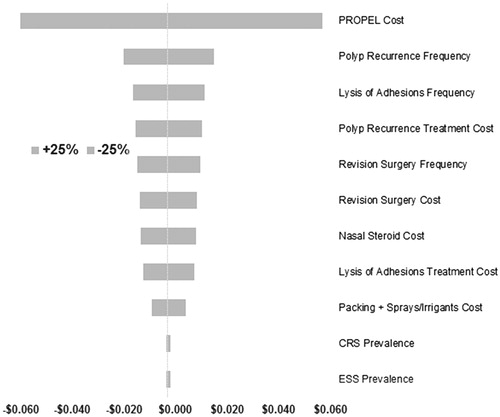
One-way sensitivity analyses were conducted by varying the reference model inputs by ±25% of its base-case value. A tornado diagram was used to visually demonstrate the resulting incremental PMPM value corresponding to the altered model input ().
Figure 2. Tornado chart of one-way sensitivity analysis for self-insured employer. Self-insured employer (base case) scenario result was −$0.003 PMPM. CRS, chronic rhinosinusitis; EES, endoscopic sinus surgery; PMPM, per member per month.
For the probabilistic sensitivity analyses, the decision tree model was used to run probabilistic Monte-Carlo simulations with 10,000 trials. The proportion of trials with PMPM cost savings was determined along with the mean cost per patient for each treatment arm.
Scenario analyses
For the scenario analyses, two different treatment patterns were examined. In the first case, it was hypothesized that Propel benefits would be consistent with the clinical trial results. Therefore, only complications occurring within the first 2 months post-ESS procedure and the likelihood of receiving a 6-month revision surgery (due to severe symptom recurrence during the early post-operative care) were considered. This scenario, by definition, would not include any complications except revision surgery during the 3–6 post-operative months. The second scenario envisioned a formulary decision of Propel being available only to the sub-population of refractory CRS patients incurring nasal polyps (CRSwNP). The prevalence of this target sub-group was assumed to be ∼30%Citation36, while the other model parameters remain unchanged.
Results
Base-case
For a US self-insured employer health plan of 1.5 million members, the model estimated the incremental PMPM impact of Propel at −$0.003 (range = −$0.408 to $0.254). Despite the upfront device cost, localized steroid delivery via Propel was expected to save the plan money through reduced healthcare utilization related to post-ESS complications. For a third party payer with less interest in labor productivity costs and the same number of lives covered, Propel’s budget impact for all members in the plan was estimated to increase costs to a US payer by $0.036 (range = −$0.331 to $0.259) PMPM.
Sensitivity analysis
One-way sensitivity analyses of the US self-insured employer model demonstrated that Propel’s budget impact was sensitive to several parameters including the: (1) cost of the implant Propel, (2) probability of polyposis recurrence requiring oral steroid treatment, (3) frequency of lysis of adhesions, and (4) cost of treatment for polyp recurrence (). In the commercial payer model (without labor productivity effects), one-way sensitivity findings did not change substantively from the self-insured employer model, thus confirming the robustness of the above conclusions.
In the probabilistic sensitivity analysis, a self-insured employer was expected to reduce PMPM costs 53.1% of the time. In 52.8% of the cases, the PMPM cost savings were expected to be greater than −$0.003 (base case result).
Scenario analysis
In the 2-month conservative scenario (scenario #1), the dollar impact of utilization of Propel following ESS was calculated to vary between $0.070 and $0.088 with and without productivity costs, respectively (). Restricting use of Propel to the high-risk sub-group of CRSwNP (scenario #2) was found to have an impact between −$0.001 to $0.011 PMPM, depending on wage loss considerations.
Table 2. Results of scenario analyses.
Discussion
Third-party payers and government agencies are increasingly requiring evidence of system cost impact to support adoption of innovative technologies. This study developed a budget-impact model to evaluate the financial impact of implementing the Propel steroid-eluting implant during ESS for CRS. The current model is specific to the US market, but the framework retains its strengths of flexibility and adaptability to various scenarios. The model structure may, thus, be adopted for other payer systems in the US or outside of the US to assess the impact of the use of BDESI (Propel) in specific populations. Our study findings suggest that using Propel during ESS for CRS may lead to potential cost savings for a self-insured employer (−$0.003 PMPM) and has minimal impact on the healthcare budget of a US third party commercial payer ($0.036 PMPM). The observed cost savings of Propel for self-insured employers are a reflection of the labor productivity gains associated with reduced job absenteeism.
Residual inflammation and recurrent polyps are common in the post-ESS period for patients with recalcitrant CRSCitation6,Citation37. This persistent symptomatology leads to repeated rounds of corticosteroids, off-label treatments, and multiple revision surgeries. According to Bajaj et al.Citation22, among those undergoing revision surgery procedures, only three out of five indicate any improvement in their symptoms; one-third undergo two revisions, and 9.5% receive three operations or more. Considering that 82% of adult CRS patients fall within the working age group (between 18–65 years old)Citation38,Citation39 and given that more than 257,000 ambulatory sinus surgery procedures are performed annually in the USCitation40, the overall labor productivity costs pertaining to the CRS refractory cases is sizable to any self-insured employer. Further highlighting this group’s burden, Rudmik et al.Citation41 found that the overall annual labor productivity cost exceeded $10,000 per refractory CRS patient.
We also evaluated the use of Propel for the high risk CRSwNP group. CRSwNP patients are especially challenging due to the greater range and burden of symptoms together with a higher post-ESS relapse rate. The reasons for the high burden of recalcitrant CRS cases are even more common in the CRSwNP groupCitation42. For these patients, there is no effective and universal cure. Frequent exacerbations and inadequate symptom control lead to augmenting the ongoing chronic treatment regimens with acute treatments, which amount to a high cumulative cost burden to manage this CRSwNP sub-type. In our analysis, offering Propel to this sub-group would lead to an incremental PMPM impact of −$0.001 to $0.011 depending on wage loss considerations.
To place Propel and its investment cost in perspective, we consider its impact relative to the overall spending by an average insurance company’s PMPM. In 2012, the American Health Policy Institute found that for all employers the average annual cost was $3430 per member per year, i.e. $285.83/monthCitation43. For large employers (1000 or more employees), PMPM was found to be $415.83. Another recent report found that the average US employer’s PMPM costs were $379.75 in 2013 for all claimsCitation44. This dollar value was projected to increase to $419.40 by 2015. While the current study’s base-case PMPM for a self-insured employer is estimated to achieve cost savings of $0.003 PMPM, if we assume that a commercial payer also shares similar PMPM burdens, then incorporating Propel would translate to a mean impact estimated to range between 0.009–0.013%. As such, Propel’s budget impact to any US employer (large or small) or a commercial payer can be considered as minimal.
Limitations
The simulation analysis used in this study was designed to represent real-world ESS care scenarios. The published clinical evidence for Propel which served as the basis for this analysis was a meta-analysis of two RCTs comparing a BDESI (Propel) to a bioabsorbable sinus implant without drug coating. The lack of any one standard post-operative treatment approach necessarily implies that some treatment protocols will differ from that included in this study (e.g. use of packing materials and/or intranasal corticosteroid therapy). Hence, our findings may not be generalizable to these situations. Moreover, comparator data used for model input values are aggregated published evidence. These input values were obtained from multiple sources in the published literature, several of which originated from small single-center studies. Thus, the two treatment arms may differ somewhat in terms of patient mix, which is another limitation of this study. Lack of sufficient published evidence for other common post-operative treatment options (e.g. spacers) may have resulted in the exclusion of other comparators from our study. In addition, the post-operative complications of interest may present themselves during regularly scheduled post-operative follow-up visits which could negate their additional visit burden as calculated in the model. Finally, our clinical data were taken from randomized trials where patient compliance is usually high. Compliance may differ in actual practice, with uncertain effects on the results. This is an important direction for further research when appropriate data become available.
Conclusions
The use of a BDESI (Propel) in refractory CRS patients following ESS is expected to yield cost savings (−$0.003 PMPM) for a self-insured employer and to have a negligible impact on the healthcare budget in the case of a commercial payer ($0.036 PMPM). The upfront cost of the implant was found to be largely offset by savings associated with reduced frequency of polyp recurrence, lysis of adhesions and their subsequent treatment.
Transparency
Declaration of funding
This study was funded by Intersect ENT, Inc. The publication of study results was not contingent on the sponsor’s approval or censorship of the manuscript.
Declaration of financial/other relationships
SRP is an employee and JAR is a consultant to CTI Clinical Trial and Consulting Services, Inc. which is a paid consultant to Intersect ENT, Inc. PJM was an employee of CTI Clinical Trial and Consulting Services, Inc. at the time the study was conducted. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Notice of correction
Please note that the article has been edited since it was first published online (21 April 2016).
Acknowledgments
No assistance in the preparation of this article is to be declared.
References
- Caulley L, Thavorn K, Rudmik L, et al. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol 2015;136:1517-22
- Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc 2013;34:328-34
- Shashy RG, Moore EJ, Weaver A. Prevalence of the chronic sinusitis diagnosis in Olmsted County, Minnesota. Arch Otolaryngol Head Neck Surg 2004;130:320-3
- Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope 2015;125:1547-56
- Rudmik L, Soler ZM. Medical Therapies for adult chronic sinusitis: a systematic review. JAMA 2015;314:926-39
- Janisiewicz A, Lee JT. In-office use of a steroid-eluting implant for maintenance of frontal ostial patency after revision sinus surgery. Allergy Rhinol (Providence) 2015;6:68-75
- Baguley C, Brownlow A, Yeung K, et al. The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int Forum Allergy Rhinol 2014;4:525-32
- Senior BA, Kennedy DW, Tanabodee J, et al. Long-term results of functional endoscopic sinus surgery. Laryngoscope 1998;108:151-7
- Rudmik L, Soler ZM, Orlandi RR, et al. Early postoperative care following endoscopic sinus surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol 2011;1:417-30
- Han JK, Marple BF, Smith TL, et al. Effect of steroid-releasing sinus implants on postoperative medical and surgical interventions: an efficacy meta-analysis. Int Forum Allergy Rhinol 2012;2:271-9
- Rudmik L, Smith TL. Economic evaluation of a steroid–eluting sinus implant following endoscopic sinus surgery for chronic rhinosinusitis. Otolaryngol Head Neck Surg 2014;151:359-66
- Brosa M, Gisbert R, Rodriguez JM, et al. Principios, métodos y aplicaciones del análisis del impacto presupuestario en el sector sanitario. PharmacoEconomics Spanish Res Articles 2005;2:65-78
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5-14
- Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016;6(1 Suppl):S22-S209
- Venkatraman G, Likosky DS, Zhou W, et al. Trends in endoscopic sinus surgery rates in the Medicare population. Arch Otolaryngol Head Neck Surg 2010;136:426-30
- Berlucchi M, Castelnuovo P, Vincenzi A, et al. Endoscopic outcomes of resorbable nasal packing after functional endoscopic sinus surgery: a multicenter prospective randomized controlled study. Eur Arch Otorhinolaryngol 2009;266:839-45
- Bugten V, Nordgard S, Skogvoll E, et al. Effects of nonabsorbable packing in middle meatus after sinus surgery. Laryngoscope 2006;116:83-8
- Chandra RK, Conley DB, Kern RC. The effect of FloSeal on mucosal healing after endoscopic sinus surgery: a comparison with thrombin-soaked gelatin foam. Am J Rhinol 2003;17:51-5
- Kastl KG, Betz CS, Siedek V, et al. Effect of carboxymethylcellulose nasal packing on wound healing after functional endoscopic sinus surgery. Am J Rhinol Allergy 2009;23:80-4
- Miller RS, Steward DL, Tami TA, et al. The clinical effects of hyaluronic acid ester nasal dressing (Merogel) on intranasal wound healing after functional endoscopic sinus surgery. Otolaryngol Head Neck Surg 2003;128:862-9
- Valentine R, Athanasiadis T, Moratti S, et al. The efficacy of a novel chitosan gel on hemostasis and wound healing after endoscopic sinus surgery. Am J Rhinol Allergy 2010;24:70-5
- Bajaj Y, Gadepalli C, Reddy T. Functional endoscopic sinus surgery: review of 266 patients. Internet J Otorhinoloaryngol 2006;6
- Chang CC, Tai CJ, Ng TY, et al. Can FESS combined with submucosal resection (SMR)/septoplasty reduce revision rate? Otolaryngol Head Neck Surg 2014;151:700-5
- Hopkins C, Slack R, Lund V, et al. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope 2009;119:2459-65
- Jakobsen J, Svendstrup F. Functional endoscopic sinus surgery in chronic sinusitis–a series of 237 consecutively operated patients. Acta Otolaryngol Suppl 2000;543:158-61
- Levine HL. Functional endoscopic sinus surgery: evaluation, surgery, and follow-up of 250 patients. Laryngoscope 1990;100:79-84
- Mascarenhas JG, da Fonseca VM, Chen VG, et al. Long-term outcomes of endoscopic sinus surgery for chronic rhinosinusitis with and without nasal polyps. Braz J Otorhinolaryngol 2013;79:306-11
- Matthews BL, Smith LE, Jones R, et al. Endoscopic sinus surgery: outcome in 155 cases. Otolaryngol Head Neck Surg 1991;104:244-6
- Mendelsohn D, Jeremic G, Wright ED, et al. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol 2011;120:162-6
- Tran KN, Beule AG, Singal D, et al. Frontal ostium restenosis after the endoscopic modified Lothrop procedure. Laryngoscope 2007;117:1457-62
- Forwith KD, Chandra RK, Yun PT, et al. ADVANCE: a multisite trial of bioabsorbable steroid-eluting sinus implants. Laryngoscope 2011;121:2473-80
- Avalere Health. Trendwatch Chartbook 2014: Avalere Health analysis of American Hospital Association annual survey data, 2012, for community hospitals. Washington, DC: Avalere Health, 2014. http://www.aha.org/research/reports/tw/chartbook/2014/14chartbook.pdf. Accessed August 28, 2015
- United States Bureau of Labor Statistics. US Medical Care Consumer Price Index. 2015. http://data.bls.gov/cgi-bin/surveymost?cu. Accessed November 25, 2015
- Donaldson A, Donatelli L, Heineman T, et al. Comparative study of postoperative steroid usage after endoscopic sinus surgery in patients with and without steroid-eluting stent placement. Poster presented at the 60th American Rhinologic Society Annual Meeting, Orlando, FL, September 20, 2014
- United States Bureau of Labor Statistics. Employment, hours, and earnings from the current employment statistics survey. http://www.bls.gov/data/. Accessed November 25, 2015
- Bhattacharyya N. Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope 2007;117:1834-8
- Lavigne F, Miller SK, Gould AR, et al. Steroid-eluting sinus implant for in-office treatment of recurrent nasal polyposis: a prospective, multicenter study. Int Forum Allergy Rhinol 2014;4:381-9
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 2014;10:1-161
- Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012: 3 p preceding table of contents, 1-298
- Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope 2010;120:635-8
- Rudmik L, Smith TL, Schlosser RJ, et al. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope 2014;124:2007-12
- Bhattacharyya N. Assessing the additional disease burden of polyps in chronic rhinosinusitis. Ann Otol Rhinol Laryngol 2009;118:185-9
- American Health Policy Institute. Health coverage cost per covered life: government vs. employment sponsored programs. Washington, DC: American Health Policy Institute, 2014. http://www.americanhealthpolicy.org/Content/documents/resources/AHPI_STUDY_Cost_Per_Covered_Life.pdf. Accessed December 1, 2015
- Truven Health Analytics. Health Leaders Media 2015 Factfile: Trends in health insurance costs. Ann Arbor, MI: Truven Health Analytics, 2015. http://healthleadersmedia.com/content/313101.pdf. Accessed December 1, 2015
- Murphy MP, Fishman P, Short SO, et al. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg 2002;127:367-76
