Abstract
Introduction: Long-term exposure to calcineurin inhibitor-based immunosuppressant (IS) therapy in liver transplant (LT) recipients is associated with renal complications. In the randomized trial H2304, everolimus + reduced-dose tacrolimus (EVR + rTAC) demonstrated equivalent efficacy and superior renal function compared to standard-dose tacrolimus.
Methods: To evaluate the cost-effectiveness of EVR + rTAC vs TAC, in de novo LT patients, a Markov model simulating both liver and kidney function was developed and estimated the long-term outcomes of IS following LT. The analysis used the Italian healthcare payer perspective.
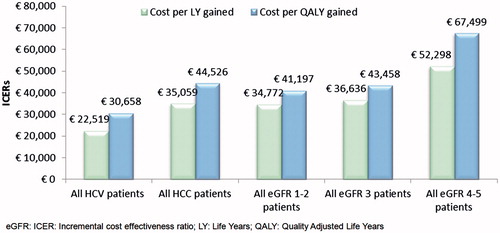
Results: Patients treated with EVR + rTAC gained on average 1.92 years and 1.62 quality-adjusted life years (QALYs). The incremental cost-effectiveness ratios (ICER) were €35,851 and €42,567 for LY gained and QALY gained, respectively. For the hepatitis-c sub-population, the ICERs decreased to €22,519 and €30,658, respectively.
Conclusion: EVR + rTAC improves survival and quality-of-life and is a cost-effective alternative to calcineurin-inhibitor monotherapy for patients requiring LT.
Introduction
Liver transplantation (LT) is a widely accepted form of therapy for patients suffering from acute liver failure, end-stage liver disease, and hepatic malignanciesCitation1. In Europe, the primary indications for LT have changed over time. In contrast to the 1980s when hepatic malignancies were a leading cause for transplantation (50%), liver cirrhosis caused by viral hepatitis and chronic alcoholism are currently the most common indications, accounting for 23% and 20% of the liver transplants, while primary hepatic malignancies represent only 16% of all liver transplantation casesCitation2. Worldwide, the number of patients receiving LT has also steadily increased over time. The European Liver Transplant Registry (ELTR; 2012) estimates that 5800 LTs are performed annually in EuropeCitation2. In 2010, the rate of LT in Italy was ∼16.69 per million populationCitation3.
Post-transplantation graft and patient survival rates have improved significantly following advances in surgical procedures and with the introduction of calcineurin inhibitors (CNI). In Europe, 1-year patient survival rates increased from only 33% before 1985 to 76% in 1990–1994 and 85% after 2004Citation2. Despite these improvements in survival rates, liver transplant recipients are still at risk from a number of complications related to surgery, disease recurrence (e.g. hepatitis-c virus [HCV] progression), and long-term use of immunosuppression (IS) regimensCitation4–9.
CNIs have been the cornerstone of IS therapy over the last 30 years, improving post-LT patient and graft survival and reducing the incidence of acute rejection (AR) episodes. However, prolonged exposure to CNIs is associated with several complications such as nephrotoxicity that can lead to chronic kidney disease (CKD), neurotoxicity, malignancies, metabolic complications, and hypertension. Studies have shown CKD to affect cumulatively ∼25% of liver transplant recipients over the first 10 years of IS therapyCitation6–11.
Thus, the current focus of post-LT care involves developing treatment strategies that help reduce the long-term complications caused by immunosuppressant strategies while effectively preventing organ rejection and graft lossCitation9,Citation10,Citation12–15.
Certican (everolimus; EVR) is a proliferation signal inhibitor (also known as mammalian target of rapamycin (mTOR) inhibitor)) that provides effective immunosuppression while protecting against renal dysfunction when used in combination with reduced doses of CNI. In the early 2000s, EVR, in combination with cyclosporine and steroids, was approved in many European countries for both kidney and heart transplants.
Efficacy and safety of EVR in combination with reduced-dose tacrolimus (TAC) was compared to standard-exposure TAC in a 24-month, multi-center, open-label randomized, controlled study (H2304)Citation16–19. This trial included de novo liver transplant recipients and was designed to assess the maintenance of renal function by allowing for the reduction or elimination of TAC early post-transplant. The extension study (H2304E1)Citation20 to the aforementioned randomized controlled trial collected data up to 36 months post-transplant. These trials concluded that patients receiving EVR in combination with reduced-dose TAC (EVR + rTAC) demonstrated non-inferior efficacy and superior renal function compared to those who received the standard-dose TAC. Furthermore, the improvements in renal function were sustained at month 24. In the extension population a similar effect from month 24 to month 36 was observed.
Based on this evidence, the use of EVR in combination with reduced-dose TAC and steroids in adult LT patients was approved by the European health authorities in 2012 and the US Food and Drug Administration (FDA) in 2013Citation9,21.
The objective of this study is to explore the cost-effectiveness of EVR + reduced-dose TAC compared to standard-dose TAC alone in de novo liver-recipients in Italy. To our knowledge, there is little evidence on the long-term economic impact of IS therapy in post-LT patients.
Methods
Model structure
A Markov state-transition model was developed in Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA) to estimate the long-term costs and effects associated with IS following LT. Based on the design of the pivotal EVR Phase III trial (study H2304), the target population consisted of a representative group of adults aged ≥18 years who received a primary allograft from a deceased donor.
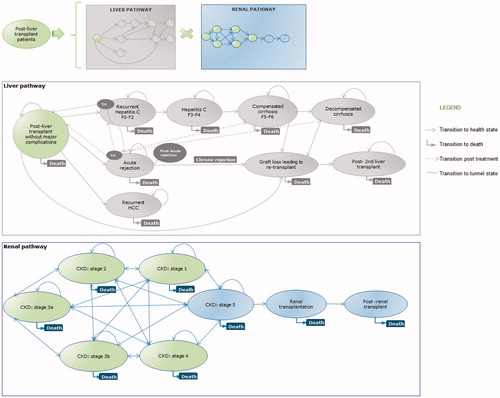
The Markov model was designed to simulate the development of post-transplant complications along two pathways: (1) liver-related: leading to graft lost and (2) kidney-related: culminating in ESRD. All patients entered the model at day of transplant and, following a run-in period of 30 days, were randomized to receive EVR + reduced-dose TAC or standard-dose TAC. All patients followed both the liver and kidney pathways (). This structure allowed all possible combinations of liver status and kidney function; which were assumed to be independent. The liver pathway was considered to be the main model pathway. Costs and utilities (or decrements) were assigned to each health state. For those experiencing renal complications, incremental costs and utility decrements were attributed and applied to those accrued in the liver pathway. The only connection between the pathways was the death status as patients dying from renal complications had to exit also the liver pathway and vice versa. Patients in need of a second LT following graft loss were absorbed into the post-LT health state and did not re-enter the model. All health states were associated with a risk of death.
The cycle length was annual, with the exception of the first year post-LT that was split into three cycles (0–3, 3–6, and 6–12 months) to allow the model to report the early complications post-LT. Half cycle correction was incorporated to compensate for the fact that in Markov models transitions are assumed to occur at the beginning or end of the cycle. In the clinical setting, patients could transition between health states at any given time, thus the half cycle correction was applied from year 2, as the three monthly cycles in year 1 were deemed sufficient to capture the transition of patients.
The analysis was based on the Italian healthcare payer perspective and included direct healthcare costs only. A lifetime horizon was considered; however, the model was built with the flexibility to allow for results over shorter time scales (i.e. 1, 2, 3, 5, 10, 20, and 30 years).
The model structure and assumptions were reviewed and validated by clinical experts.
Model inputs
Efficacy
Most efficacy data were obtained from the H2304 study and, wherever possible, estimates from published literature were used to populate the model. Data on patient response to the treatment of acute rejection (AR) by level of severity were obtained from consultations with clinical experts.
The risks of AR, change in renal function, and hepatitis C (HCV) progression were specific to treatment. For AR, odds ratios (OR) for each treatment regimen were calculated based on the analysis of the H2304 and the H2304E1 patient level data (PLD).
Post-LT, HCV recurrence was assumed to occur in the first 3 months and disease progression was characterized based on the following three transitions post-LT: (1) mild-to-moderate HCV, (2) moderate HCV to compensated cirrhosis, and (3) compensated cirrhosis to decompensated cirrhosis. Data on the potential effect of the EVR + rTAC regimen and the standard-dose TAC regimen on HCV progression were obtained from the H2304 clinical trial.
The H2304 PLD were used to derive the transition probabilities between CKD stages for EVR + rTAC and standard-dose TAC alone arms for the first 3 years. The ORs were then assessed for each model cycle.
Treatment-emergent adverse events (TEAEs), associated with the two IS regimens, were also incorporated in the model. The model assumed TEAEs (with the exception of renal dysfunction) did not occur after year 3 due to the lack of clinical data. In the absence of long-term data, the analysis assumed that death due to graft loss was not treatment-specific.
Transition probabilities
The early transition probabilities (TPs; i.e. for the first 3 years) associated with hepatic complications were derived using the H2304 and the H2304E1 PLD and supplemented with the published literatureCitation22. As data on the risk of AR among patients experiencing liver complications such as HCV fibrosis and cirrhosis were not available, conservative assumptions were made following consultations with the clinical experts.
In terms of the kidney pathway, TPs within the 36 months following liver transplantation were derived from H2304 and the H2304E1 PLD. For subsequent years of the model, TPs related to progressive kidney failure were derived from UNOS database analysisCitation23. The TP for EVR + reduced TAC were calculated using the OR derived from the trial data. However, as no patient experienced CKD stage 5 within the H2304 and the H2304E1 timeframe, the annual probability of patients requiring a renal transplant was assumed to be 0.02%.
Resource use and costs
A focused search of the literature was conducted to identify Italian costs associated with the liver and renal pathways. This was supplemented by local expert opinion.
Drug costs for all of the treatment strategies considered in the model were obtained from FARMADATI ITALIA (2013; ).
Table 1. Unit cost per milligram.
Costs associated with monitoring following transplant included outpatient and inpatient care, and laboratory tests. Resource use estimates were based on clinical opinion.
The costs associated with each complication ( [t]insert near here[/t]) were obtained from the literature, and these costs were independent of the monitoring costsCitation24. All costs were adjusted to 2014 prices using the Italian ISTAT (The National Institute of Statistics) consumer price index for medical care.
Table 2. Estimated annual health states costs (EUR; €).
Utilities
The baseline utility for the study population is the value associated with post-LT without major complications. Quality-of-life data were not collected as part of H2304, thus utility values were based on baseline utility estimates of EQ-5D scores from Russell et al.Citation25. The estimates were derived through calibration of the model utilities to match the values reported in Russell et al. (early post-transplant =0.746; intermediate post-transplant =0.765; late post-transplant =0.817), as described in Citation25. Quality-of-life is affected each time patients experience complications (hepatic and/or renal). Thus, the utilities associated with the liver health states were combined with the baseline utilities using the multiplicative approach and each kidney complication and adverse event were then applied as decrements using a subtractive approach. These values were taken from the literature.
Table 3. Health state utilities post-liver transplantation.
Model outcomes
The main outcome of consideration was the incremental cost-effectiveness ratio (ICER). The analyses also considered other effectiveness outcomes such as life years (LYs) and quality-adjusted life years (QALY). The ICER is measured by comparing the value to a threshold level of willingness to pay (WTP). According to the AIES (Italian Association of Health Economics) a technology within the range of €25,000–€40,000 per QALY is considered to be valuableCitation27. In addition, clinical outcomes such as the number of AR and graft losses avoided were also examined, as well as the economic costs associated with renal and liver complications.
Sensitivity analyses
In order to assess the uncertainty in the results, deterministic and probabilistic sensitivity analyses were carried out. In the deterministic sensitivity analysis (DSA) the following inputs were varied: transition probabilities (± 25%), utilities (± 25%), health state costs (± 20%), drug costs (± 20%), AE costs (± 20%), and discounting (3%). In terms of the efficacy parameters, the proportion of patients treated with HCV was decreased from 50% in the base case to 25% in the DSA. For those patients who experienced a mild acute rejection, no treatment of these patients was also investigated. In the clinical study report two methods were used to estimate the OR associated HCV progression on reduced dose tacrolimus. In the base case a value of 0.37 was applied and the DSA examined an alternative value of 0.68.
In the probabilistic sensitivity analysis (PSA) TPs, utilities, health state costs, drug costs, and AE costs were varied. A beta distribution was applied to the TPs and health state utilities, a log-normal for AE utilities, and a gamma distribution for all cost parameters.
Sub-group analyses were also conducted. The following patient groups were investigated based on liver disease background and renal function at baseline: HCV, hepatocellular carcinoma (HCC), CKD stage 1–2, 3a–3b, and 4–5.
Results
Base case
The model predicts that, with a mean life expectancy of 18 years, patients treated with EVR + reduced TAC would gain 1.92 LY and 1.62 QALY vs TAC alone ( near here[/t]), with a cost per LY gained of €35,851 and the cost per QALY gained close to the upper bound of the AIES threshold (€42,567/QALY). Pharmacological costs represented the major share of the total costs for post-LT patients. The higher IS cost of EVR + reduced TAC was slightly offset by lower management costs. Costs associated with renal complications were more favorable in the EVR arm, accounting for 15% of the total costs compared to 21% in the TAC arm; this was explained by the lower number of cases of severe CKD (stage 3–5) in the EVR arm. EVR + rTAC was also associated with a considerable reduction in the frequency of acute rejections (20%), resulting in a slight cost offset in the EVR arm.
Table 4. Lifetime base case results.
The EVR regimen demonstrated that it was a favorable strategy in the sub-group of patients with liver complications at baseline. For the HCV sub-group, the incremental cost per LY and QALY gained decreased to €22,519 and €30,658, respectively ().
Sensitivity analyses
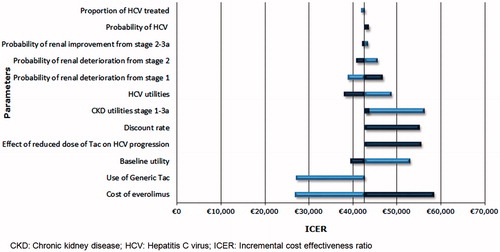
The tornado diagram in illustrates the difference from the base case in the lifetime cost per QALY observed for a series of sensitivity analyses performed on key parameters. The results of the DSA demonstrated that the most sensitive parameters were the price of EVR, the use of generic TAC, discount rate, the utilities associated with baseline and CKD stages (1–3a), as well as the effect of reduced-dose TAC on HCV progression.
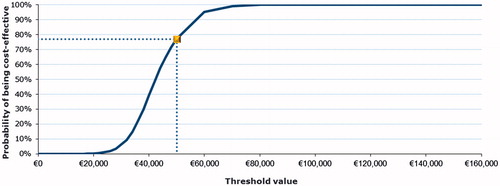
Probabilistic sensitivity analysis showed that the EVR strategy remained costlier, but also more effective than TAC alone across the simulations. The cost-effectiveness acceptability curve () shows a 77% probability of ICERs remaining below the €50,000 threshold. The probability of not exceeding the threshold of €40,000 was 39%.
Discussion
The long-term use of CNI-based IS regimens is associated with several complications that have a significant impact on patient and graft survivalCitation2,Citation4,Citation6–10. Thus, there is a need to develop treatment strategies that can reduce CNI-induced complications while effectively preventing organ rejection and graft lossCitation9,Citation10,Citation12–15.
EVR is an alternative IS agent that was approved by the FDA and the European health authorities for LT9,21. EVR being a proliferation signal inhibitor (mTOR inhibitor) has a different pharmacological profile to the CNIs and, hence, provides effective immunosuppression with less CNI-related adverse effects if combined with reduced exposure to CNIs. Phase III trials have shown that patients receiving EVR with reduced dose TAC experienced non-inferior efficacy and superior renal function compared to those who received the standard-dose TAC16,17,20.
Furthermore, our cost-effectiveness analysis has shown that the EVR with reduced dose TAC regimen is associated with gains in LY (1.92) and QALY (1.62) years vs standard-dose TAC. Everolimus with reduced dose TAC was associated with substantially lower renal complications and ARs. Although the ICER is close to the upper bound of the AIES threshold (€42,567/QALY) for the overall population, it is worth noting that EVR with reduced dose TAC has a favorable cost-effectiveness profile compared to the standard-dose TAC regimen among patients with HCV who received a de novo liver transplantation. In Europe, these sub-groups account for ∼40% of all transplantation cases.
Our analysis has several strengths. First, the efficacy data vs standard-dose TAC is derived from a head to head clinical trial and is robust in sensitivity analyses considered in the model. Second, patient level data were also used, which increases the validity of the model. It also allowed for the exploration of sub-group analyses. The base case results were found to be robust in several sensitivity analyses that were conducted. Finally, to our knowledge this is the first cost-effectiveness model investigating post-liver transplantation and the impact of change in renal function on patient survival.
This analysis is not without limitations. Due to a lack of data in the literature or in the study some assumptions were applied for transition probabilities and mortality. At the time of writing only one published model existed measuring fibrosis progression post-liver transplant. Therefore, many assumptions were applied for acute rejection and the progression of HCV and HCC. As the transition probabilities within CKD stages were not available in the UNOS database after 5 years post-transplant, the model assumed these parameters remained constant from year 5 onwards. There was also limited published evidence on mortality rates relevant to certain health states (i.e. compensated cirrhosis, HCC, acute rejection, CKD stage 1 and death following renal transplant) considered in the model, and long-term TEAEs beyond the 3 years trial period.
Transparency
Declaration of funding
This study was funded by Novartis Pharma.
Declaration of financial/other relationships
JG and AR are employees of Novartis Pharma. RC, VD, and FB are employees of Mapi Group, who received funding from Novartis Pharma to conduct this study. PDS was the lead investigator for the randomized controlled trials of everolimus and received funding from Novartis Pharma. JFR has received compensation for consultancy work from Novartis Pharma to conduct this study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors take full responsibility for the content of this contribution and would like to thank Dr. William Irish of CTI Clinical Trial and Consulting Services for performing the UNOS database analysis.
References
- Varma V, Mehta N, Kumaran V, Nundy S. Indications and contraindications for liver transplantation. Int J Hepatol 2011;2011:121862
- Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-88
- Kasiske BL, Skeans MA, Leighton TR, et al. OPTN/SRTR 2011 Annual Data Report: international data. Am J Transplant 2013;13(Suppl 1):199-225
- Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999–2008. Am J Transplant 2010;10(4 Pt 2):1003-19
- McDiarmid SV, Colonna JO, Shaked A, et al. A comparison of renal function in cyclosporine- and FK-506-treated patients after primary orthotopic liver transplantation. Transplantation 1993;56:847-53
- Patel HK, Patel A, Abouljoud M, et al. Survival after liver transplantation in patients who develop renal insufficiency. Transplant Proc 2010;42:4167-70
- Pham PT, Pham PC, Wilkinson AH. Management of renal dysfunction in the liver transplant recipient. Curr Opin Organ Transplant 2009;14:231-9
- Pillebout E, Nochy D, Hill G, et al. Renal histopathological lesions after orthotopic liver transplantation (OLT). Am J Transplant 2005;5:1120-9
- Ganschow R, Pollok JM, Jankofsky M, Junge G. The role of everolimus in liver transplantation. Clin Exp Gastroenterol 2014;7:329-43
- Aberg F, Isoniemi H, Hockerstedt K. Long-term results of liver transplantation. Scand J Surg 2011;100:14-21
- Fabrizi F, Dixit V, Martin P, Messa P. Chronic kidney disease after liver transplantation: Recent evidence. Int J Artif Organs 2010;33:803-11
- Benten D, Staufer K, Sterneck M. Orthotopic liver transplantation and what to do during follow-up: recommendations for the practitioner. Nat Clin Pract Gastroenterol Hepatol 2009;6:23-36
- Gelson W, Hoare M, Dawwas MF, et al. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation 2011;91:1240-4
- Moon DB, Lee SG. Liver transplantation. Gut Liver 2009;3:145-65
- Pruthi J, Medkiff KA, Esrason KT, et al. Analysis of causes of death in liver transplant recipients who survived more than 3 years. Liver Transpl 2001;7:811-15
- De SP, Nevens F, De CL, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:3008-20
- Saliba F, De SP, Nevens F, et al. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant 2013;13:1734-45
- Efficacy and safety of concentration-controlled everolimus to eliminate or to reduce tacrolimus compared to tacrolimus in de novo liver transplant recipients [Internet]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2008. https://clinicaltrials.gov/ct2/show/NCT00622869?term=h2304&rank=2. Accessed November 9, 2015
- Extension study to evaluate the long-term efficacy and safety of everolimus in liver transplant recipients [Internet]. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2010. https://clinicaltrials.gov/ct2/show?term=h2304&rank=1. Accessed November 9, 2015
- Duvoux C, Saliba F, Kaiser G, et al., editors. Efficacy and safety of everolimus with reduced tacrolimus in de novo liver transplant recipients: Long-term results from the H2304E1 study. World Transplant Congress; July 26, 2014; San Francisco; 2014
- Novartis. Media Release: Novartis drug Zortress® is first in over a decade approved by FDA to prevent organ rejection in adult liver transplant patients. Novartis; 2013 [updated 2013] http://asts.org/docs/default-source/resource/novartis-drug-zortress(r)-is-first-in-over-a-decade-approved-by-fda-to-prevent-organ-rejection-in-adult-liver-transplant-patients.pdf?sfvrsn=0
- Terrault N. Liver transplantation in the setting of chronic HCV. Best Pract Res Clin Gastroenterol 2012;26:531-48
- United Organ Sharing Network [Internet]. https://www.unos.org/data/
- Ruggeri M, Coretti S, Gasbarrini A, Cicchetti A. Economic assessment of an anti-HCV screening program in Italy. Value Health 2013;16:965-72
- Russell RT, Feurer ID, Wisawatapnimit P, Pinson CW. The validity of EQ-5D US preference weights in liver transplant candidates and recipients. Liver Transpl 2009;15:88-95
- Farmadati Italia 2013. Accessed: 15th June 2013. http://www.farmadati.it/
- Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. PharmacoEconomics Italian Research Articles 2009;11:83-93
- Camma C, Petta S, Enea M, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology 2012;56:850-60
- Rufat P, Fourquet F, Conti F, et al. Costs and outcomes of liver transplantation in adults: a prospective, 1-year, follow-up study. GRETHECO study group. Transplantation 1999;68:76-83
- Cicchetti ARM, Codella P, Ridolfi A. I costi socio-sanitari dell’insufficienza renale cronica. Farmeconomia e Percorsi Terapeutici 2011;12:21-8
- Villa G, Rodriguez-Carmona A, Fernandez-Ortiz L, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011;26:3709-14
- Sullivan SD, Craxi A, Alberti A, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics 2004;22:257-65
- Das A. Cost-effectiveness of different strategies of cytomegalovirus prophylaxis in orthotopic liver transplant recipients. Hepatology 2000;31:311-17
- Gorodetskaya I, Zenios S, McCulloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 2005;68:2801-8
- McLaughlin K, Manns B, Nickerson P. The routine use of high-resolution immunological screening of recipients of primary deceased donor kidney allografts is cost-effective. Transplantation 2006;81:1278-84
- Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996;50:235-42