Abstract
Background: Cancer cachexia is a debilitating condition and results in poor prognosis. The purpose of this study was to assess hospitalization incidence, patient characteristics, and medical cost and burden of cancer cachexia in the US.
Methods: This study used a cross-sectional analysis of the Nationwide Inpatient Sample (NIS) for 2009. Five cancers reported to have the highest cachexia incidence were assessed. The hospitalization incidence related to cachexia was estimated by cancer type, cost and length of stay were compared, and descriptive statistics were reported for each cancer type, as well as differences being compared between patients with and without cachexia.
Results: Risk of inpatient death was higher for patients with cachexia in lung cancer (OR = 1.32; CI = 1.20–1.46) and in all cancers combined (OR = 1.76; CI = 1.67–1.85). The presence of cachexia increased length of stay in lung (IRR = 1.05; CI = 1.03–1.08), Kaposi’s sarcoma (IRR = 1.47; CI = 1.14–1.89) and all cancers combined (IRR = 1.09; CI = 1.08–1.10). Additionally, cachectic patients in the composite category had a longer hospitalization stay compared to non-cachectic patients (3–9 days for those with cachexia and 2–7 days for those without cachexia). The cost of inpatient stay was significantly higher in cachexic than non-cachexic lung cancer patients ($13,560 vs $13 190; p < 0.0001), as well as cachexic vs non-cachexic cancer patients in general (14 751 vs 13 928; p < 0.0001).
Conclusions: Cachexia increases hospitalization costs and length of stay in several cancer types. Identifying the medical burden associated with cancer cachexia will assist in developing an international consensus for recognition and coding by the medical community and ultimately an effective treatment plans for cancer cachexia.
Introduction
Cancer cachexia, also known as cancer-related wasting syndrome and cancer anorexia-cachexia syndrome (CACS), is a debilitating condition in which patients experience involuntary weight loss (predominately skeletal muscle and sometimes fat), that can be driven through reduced appetite, increased energy expenditure, and/or inflammatory pathways, resulting in poor prognosis. The syndrome can be considered a co-morbidity of cancer patients and may be the leading cause of 20–30% of cancer deathsCitation1–6. An estimated 15–50% of cancer patients (depending on the cancer type and stage) experience cachexia, with some reports suggesting that as high as 80% of patients with certain cancers experience cachexia. The presence of this co-morbid condition is associated with diminished quality-of-life, poor response to chemotherapy, poor surgical outcomes and poor clinical outcomes in cancer patientsCitation5,Citation7–10.
While historic definitions of cachexia across chronic conditions have varied, a 2011 international consensus statement on cancer cachexia emphasized three diagnostic criteria: (1) unintentional weight loss (>5% over past 6 months in absence of simple starvation); (2) weight loss >2% when BMI was <20 kg/m2; and (3) appendicular skeletal muscle index consistent with sarcopenia and any weight loss >2%Citation11. Of note, the inability to prevent weight loss through nutritional interventions is one of several important distinctions between cancer cachexia and simple starvationCitation11. However, weight loss alone does affect cancer survivalCitation12. The co-occurrence of both cancer and cachexia ultimately weakens the patient’s opportunity to recover. LeBlanc et al.Citation1 demonstrated that patients with non-small cell lung cancer and classified with CACS experienced a greater decline in quality-of-life and survival than those not diagnosed with CACS. Other reports also stated that cancer-cachexia patients experienced decreases in quality-of-life and ability to function, resulting in negative impact of chemotherapy treatmentCitation5,Citation7,Citation8. Furthermore, there was increased mortality in patients with muscle wasting that underwent surgical treatment for adenocarcinoma or hepatocellular carcinoma relative to cancer patients without muscle wastingCitation5,Citation9,Citation10. Cancer treatment strategies (surgery, radiation, chemotherapy) may promote the risk for developing cachexia.
The relationship between cancer and cachexia needs to be fully elucidated so as to develop effective therapeutic strategies that consider this relationship. Patient characteristics, healthcare utilization, and cost of cancer cachexia are not well characterized in the literature. The purpose of this study was to assess hospitalization incidence, patient characteristics and inpatient death prediction, healthcare utilization, cost, and medical burden of cancer cachexia in the US.
Materials and methods
This study used a cross-sectional analysis of the 2009 Nationwide Inpatient Sample (NIS) (Healthcare Cost & Utilization Project, Agency for Healthcare Research & Quality [AHRQ]), which contains a 20% sample of hospital admissions occurring in non-federal community hospitals in the US. We first identified patients with any cancer diagnosis, and further categorized patients by specific cancer site, using International Classification of Diseases, 9th Revision (ICD9), Clinical Modification Diagnoses codes. In addition to the composite category of all cancers, we focused on the five cancers that were reported in a recent study to have the highest hospitalization incidence of cachexia: lung, esophageal, stomach, Kaposi’s sarcoma, and pancreaticCitation13. Additionally, we included a composite “any cancer2 category for patients with any form of cancer (including lung, esophageal, stomach, Kaposi’s sarcoma, and pancreatic, as well as any other form of cancer based on ICD-9 diagnosis codes). Each of these cancer populations were then categorized as having cachexia or not having cachexia. Due to poor consensus in defining cachexia, we used the diagnosis codes as seen in Fox et al.’sCitation14 paper, which were: cachexia (ICD9 799.4), any diagnosis for loss of weight (ICD09 783.21), anorexia (ICD9 783.0), or being underweight (ICD9 783.22). Those not having indications for any of these four conditions were categorized as non-cachexic. We recognize that the above criteria is not exhaustive and that other unique identifiers of cachexia may have not been capturedCitation14.
Cachexia hospitalization incidence, patient characteristics, and hospital characteristics were described independently for each cancer category. Patient characteristics included age, gender, comorbidities, type of insurance, income, discharge status and loss of function. Discharge status is a measure of where the patient went after leaving the hospital and is collected on a standard UB-04 hospital form. Discharge status includes discharged to skilled nursing, hospice, home, rehabilitation, death, and other. Loss of function is a composite measure provided by AHRQ for each admission. The measure is created by using the severity of illness and risk of mortality sub-classes of the All Patient Refined Diagnosis Related Groupings Complexity Sub-class system (APR-DRG)Citation15,Citation16. Hospital characteristics included region, location, teaching status, and ownership status.
Measures of central tendency, including frequencies, means, and medians were calculated. Bivariate statistical analysis comparing cancer patients with and without cachexia were performed using Chi-square tests for categorical variables and t-tests for continuous variables. We compared the cost and length of stay for those cancer patients who had a diagnosis of cachexia to those who did not in each cancer category. To measure predictors for increased length of stay, six separate negative binomial models were used to estimate incidence rate ratios and the corresponding 95% confidence intervals, while controlling for hospital and patient characteristics. In conjunction with the negative binomial models, six generalized linear models (GLM) were used to estimate predicted cost of cachexia within each cancer category using a log link with the gamma family distribution, while controlling for hospital and patient characteristics and death. All analyses were conducted using SAS 9.3 (Cary, NC).
Results
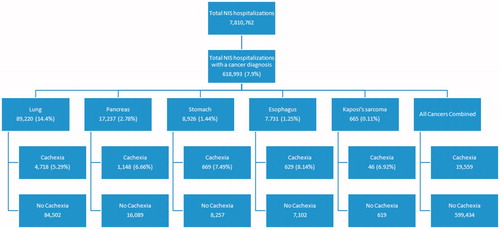
Nearly 8% of all US hospitalizations in 2009 had cancer as an admission diagnosis (). Lung cancer was the predominant admission diagnosis amongst the five specific cancer sites selected for this study (14.4% of admissions). Of lung cancer admissions, 5.29% also carried a diagnosis for cachexia as a co-morbidity, equaling 4718 admissions. Kaposi’s sarcoma was the cancer type with the highest evidence of cachexia as comorbidity (6.92%; 46 out of 665 admissions) ().
Sample weights provided by the NIS were applied to the incidence of cachexia across the five sites/types of cancer in our data, as well as an all cancers combined category. Lung cancer had the highest incidence of cachexia, followed by cancers in the digestive tract and finally Kaposi’s sarcoma. National estimates for hospitalization incidence with cachexia in the five sites/types of cancer ranged from 228 in the population with Kaposi’s sarcoma to 23 781 in the population with lung cancer ().
Table 1. Population and demographic information and median hospitalization characteristics.
Inpatient death was higher in the population with cachexia when compared with those without, across all site/types of cancers studied (). The greatest difference in percentage of admissions indicating inpatient death was seen in the all cancers combined category (11.25% in admissions with cachexia and 5.68% in admissions without cachexia).
Cachexia was more abundant in males across all cancer site/types, with the greatest gender difference seen in the population with Kaposi’s sarcoma; 98% of Kaposi’s sarcoma patients with cachexia were male. Regionally, the South had the highest incidence of cachexia diagnoses across all cancer sites, followed by the Midwest. Almost 46% of lung cancer admissions from the South had cachexia, and 42% of admissions in the all cancer combined group from the South had cachexia. Patients receiving treatment from urban hospitals had the majority of cachexia diagnoses across all cancer sites/types.
On average, patients with cachexia across all cancer sites/types experienced more severe loss of function than those without. Almost 41% of lung cancer admissions experiencing major loss of function had cachexia, while 28% of lung cancer admissions experiencing major loss of function did not have cachexia. Similarly, almost 40% of pancreatic cancer admissions, 41% of stomach cancer admissions, 37% of esophageal cancer admissions, and 39% of all cancers combined admissions experienced major loss of function with cachexia. Most patients with cachexia (39% in lung, 47% in pancreatic, 41% in stomach, 42% in esophageal, 34% in Kaposi’s sarcoma) were observed in the highest household income group ($48 000 or more per year) across all cancer sites/types ().
Across all cancer site/types, patients with cachexia had a higher number of co-morbidities than those without. Median inpatient hospitalization costs were significantly higher for patients with cachexia in lung cancer (1.03-fold; p < 0.0001) and in all cancers combined (1.06-fold; p < 0.0001) relative to those patients without cachexia ().
Risk of inpatient death was higher for patients with cachexia in lung cancer (OR = 1.32, CI = 1.20–1.46) and in all cancers combined (OR = 1.76, CI = 1.67–1.85). When comparing age categories of cancer patients, almost all categories of age were less likely to die during their inpatient stay relative to the reference group of those aged 85 and older. Males were at greater risk of dying during their inpatient stay than females in lung cancer and all cancers combined (Lung: OR = 1.16, CI = 1.11–1.22, all cancers: OR = 1.02, CI = 1.00-1.05). Urban hospitals consistently had decreased risk of inpatient death in patients compared to rural hospitals. The ability of the most common co-morbid variables to predict inpatient death was variable by cancer site. Of interest is the reduction in risk when heart failure was present in lung and all cancers combined (OR = 0.67, CI = 0.81–0.93 and OR = 0.84, CI = 0.81–0.87, respectively) ().
Table 2. Logistic model predicting the odds of inpatient death.
The presence of cachexia increased length of stay in lung (IRR = 1.05, CI = 1.03–1.08), Kaposi’s sarcoma (IRR = 1.47, CI = 1.14–1.89) and all cancers combined (IRR = 1.09, CI = 1.08–1.10) (). Men with lung (IRR = 0.97, CI = 0.96–0.98), pancreas (IRR = 0.96, CI = 0.94–0.98), stomach (IRR = 0.94, CI = 0.91–0.98), esophageal (IRR = 0.93, CI = 0.89–0.97), and all cancers combined (IRR = 0.98, CI = 0.98–0.99) had a shorter length of stay, compared to their female counterparts. The regional variable showed similar results to the death analysis, with most of the regions at decreased risk for increased length of stay compared to the Northeast. Hospital location had the opposite effect on increased length of stay, with urban hospitals having longer stays than rural hospitals. Those patients with increased loss of function had increased lengths of stay in all cancer sites except Kaposi’s sarcoma. Unlike the inpatient death analysis, our models predicting length of stay showed a gradient in the responses of loss of function levels on length of stay across all cancer sites/types studied. Moderate, major, and extreme loss of function seemingly predicted increased in-patient stay when compared to minor loss of function. For example, in lung cancer moderate loss of function (IRR = 1.21, CI = 1.18–1.25), major loss of function (IRR = 1.62, CI = 1.58–1.67) and extreme loss of function (IRR = 2.25, CI = 2.18–2.32) were all greater than minor loss of function. Co-morbid conditions showed similar results to the analysis of inpatient death, although with noticeably lower measures of effect ().
Table 3. Negative binomial model predicting risk for increased patient stay.
Discussion
In this project, we used the NIS from 2009 to study coding prevalence and hospital information for cancer cachexia in the five cancer types seen to have the highest incidence of cachexia.
Of the 618 993 cancer cases seen in the 2009 NIS, 3.16% or 19 559 cases had co-morbid cachexia. This is similar to findings reported by Fox et al.Citation14, whereby ∼2.4% of a cancer population was considered cachectic using the ICD-9 diagnostic code of 799.4 with a retrospective database study of electronic medical records of over 8000 cancer patients. Of note, expanding the cachexia diagnosis in that study to also include anorexia, abnormal weight loss, or feeding difficulties increased cachexia prevalence to 5.5%, and diagnosing based on ≥5% weight loss increased cachexia prevalence to 14.7%Citation14. Our current study also expanded cachexia diagnosis from 10 757 hospitalizations (ICD9 799.4) to 19 559 hospitalizations (ICD 799.4, 783.21, 783.0, and 783.22). Including the additional ICD codes allowed us to increase the sensitivity of cachexia diagnosis and, therefore, 8802 additional hospitalizations were captured. These data highlight how cachexia is under-recognized and under-coded by the medical community. This may be due to the absence or lack of screening protocols, standardized methods for recording weight history, and diagnostic criteriaCitation17. However, there are reports that ∼17.4% of cancer patients may experience ≥5% and <10% weight loss within 6 months of diagnosisCitation18. This suggests that the ICD9 codes used in this study may under-estimate the occurrence of a studied attribute.
Cachexia has been observed to be prevalent in lung cancer patients undergoing chemotherapy, since ∼70% of lung cancer patients exhibited weight loss and 79% displayed significant loss of muscle massCitation19. Weight loss alone can be considered a burden to the survival of cancer patients. Grivaux et al.Citation12 reported that 43.7% of lung cancer patients who lost 10 kg or more died within 3 months of diagnosis. Although not standardized, the characteristics of cancer cachexia have been associated with increased inflammation, decreased caloric intake, asthenia, anorexia, anemia, and fatigueCitation3,Citation15. The mechanism of the cachexia development may be attributable to dysfunctional metabolic and endocrine systems, increased inflammation, and protein catabolism resulting in decreased skeletal muscle mass. Since 25–50% of patients diagnosed with non-small cell lung cancer are malnourishedCitation1, decreased caloric intake may be a cause of cachexia in lung cancer patients. In addition, inflammatory-induced activation of catabolism pathways such as ubiquitin proteasome pathway in lung cancer patients resulting in apoptosis of muscle fibers may be another contributor to the loss of body weight and lean muscle mass in cachectic lung cancer patientsCitation16. Of note, the loss of body weight and lean mass are key criteria in the international consensus definition of cancer cachexiaCitation11 that may not be considered in the current ICD-9 code for cachexia.
Population demographics seen in follow the expected cancer distributions, with lung cancer occurring more in the South region of the US and Kaposi’s sarcoma occurring most often in men. However, the preponderance of observations originating from the South may be an artifact of oversampling from the region in the sampling frame.
In each cancer type, the patients with cachexia had greater loss of function in the “major” or “severe” categories. The increased severity coincided with increased length of stay for cachexia and increased costs in every cancer category (). The binomial model predicting length of stay showed similar results with cachexia being a significant contributor in lung cancer, Kaposi’s sarcoma, and other cancer sites ().
In our previous researchCitation13, we found the average difference in the cost of inpatient care between cachexic and non-cachexic patients to be $4641.30. In the present study, we find the difference to be much smaller between cachexic and non-cachexic cancer patients (e.g. $14 751 vs $13 928 for cachexic and non-cachexic patients, respectively, with any form of cancer, a difference of less than $1000). There are a number of reasons that may explain the difference in findings between studies. The Arthur et al.Citation13 study compared cachexia vs non-cachexia medical burden to all diseases which included heart failure, HIV, Septicemia, and others, while the current study only investigated the disease of cancer. In addition, in this current study, we present adjusted costs to control for differences in patient and hospital characteristics. In our previous study, we reported unadjusted costs. However, there are other important characteristics which drive the cost differences between studies. In our original study, we found that cachexic patients stayed, on average, 3 days longer than non-cachexic patients. In the present study, there was no such difference between cachexic and non-cachexic patients (for lung, pancreas, and stomach cancers the average length of stay was statistically the same between patient groups; for esophageal cancer, Kaposi’s sarcoma, and all cancers, combined, the difference between patient groups was only 1 day); thus, the difference in cost of inpatient care between studies is likely driven, at least in part, by differences in lengths of stay between groups. Finally, it is worth noting that, in our original research, far more cachexic than non-cachexic patients had major (52.60% vs 21.26%) and extreme (29.76% vs 6.27%) losses of function—a major driver of costs. In contrast, there was a less pronounced imbalance in major (e.g. 38.70% vs 24.25% for all cancers combined) or extreme (e.g. 14.86% vs 8.06% for all cancers combined) losses of function between cachexic and non-cachexic patients in our current study.
Another key finding of the present study is that cachexia was a significant predictor of inpatient death in lung cancer as well as other cancer types (). This is an important finding, as cachexia significantly increased the odds of death when controlling for loss of function and the top five other co-morbid conditions seen in cancer patients.
For most of the cancers examined in our dataset, we found cancer patients with co-morbid cachexia are hospitalized at a younger age, have a greater number of co-morbid conditions, and incur greater charges during hospital stays of the same duration. In addition, the literature states that there is a high correlation of muscle loss and mortality in patients with cancer who underwent surgery relative to the cancer patients who did not have muscle loss and who underwent surgeryCitation5,Citation9,Citation10. These insights indicate that cachexia patients may progress through their disease faster than their non-cachectic peers and potentially have worsened outcomes. This agrees with the data reported by LeBlanc et al.Citation1, who observed that lung cancer patients with cachexia experienced an accelerated rate of decline than cancer patients without cachexia, including shorter survival, worse physical function, and poorer quality-of-life.
This study was limited by the two main factors of admission data and inherent issues of diagnosis in cachexia. First, admission data only records information that was coded during the hospital stay. The information lacks insight into aspects that are not claim-related, such as stage of cancer, which may be important to understand these patients. In addition, a limitation of admission data is the validity of reporting ICD-9 codes in which there may be inaccurate representation of a population due to lack of reporting a codeCitation20,Citation21. An example related to this paper’s analysis of changes in weight and under-estimation of inpatient recordings is seen by Woo et al.Citation21 and Golinvaux et al.Citation20. Woo et al.Citation21 found that, while 20.4% of inpatient discharges were measured to be obese, only 1.7% of those inpatients were diagnosed with obesity using the ICD-9 codes for “obesity” or “overweight”. Golinvaux et al.Citation20 reported an ICD-9 code sensitivity of 0.19 and 0.48 for “obese” and “morbidly obese”, respectivelyCitation20. This misidentification of body weight changes could result in an under-representation of cachexic patients in our study. Associated with this is the low percentage of cancer patients identified as cachexic. Although the ICD9 codes used in this study resulted in a similar identification of cancer cachexic patients, as reported by Fox et al.Citation14, relative to reports that measured weight loss of patients, the cancer cachexic patients may be considered too lowCitation18. This discrepancy is due to the reliance of admissions reporting and is a limitation of using ICD9 coding data. Second, drug information was not present, which could have elaborated upon disease severity as well as increased costs. In addition, the ICD-9 codes used include cachexia as well as other aspects related to weight loss and anorexia. Although starvation should be a separate aspect of cancer survival than cachexia, the ICD-9 codes used for this study were not able to discriminate between starvation and cachexia. Future studies will include other defining aspects of cachexia that include drug information to provide in-depth characterization of cachexia. Importantly, missed diagnosis of cachexia must also be considered. As noted above, cachexia is known to be under-codedCitation14,Citation17. Although the results presented above are significant, the bias introduced by the undiagnosed cachexia patients may mask the true impact and absolute value of the problem of cachexia.
Future studies will include studying the relationship of disease state and onset of cachexia and determine if pre-cachexia influences the rate of cancer stage progression.
Conclusion
The overall impact of this study has been to further explore cancer-related cachexia on a national level. We have seen that cachexia patients have more co-morbid conditions and cost more than their peers of the same cancer type. The next step is to progress further into each cancer with a different data set capable of accurately identifying the presence/absence of cachexia to understand the impact of cachexia relative to stage of cancer.
Transparency
Declaration of funding
This work was supported by the Helsinn Group, a cancer care company.
Declaration of financial/other relationships
The terms of this arrangement have been reviewed and approved by the University of North Carolina at Charlotte in accordance with its policy on objectivity in research. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- LeBlanc TW, et al. Correlation between the International Consensus Definition of the Cancer Anorexia Cachexia Syndrome (CACS) and Patient-Centered Outcomes in Advanced Non-small Cell Lung Cancer. J Pain Symptom Manag 2015;49:680-9
- Muscaritoli M, et al. Cachexia: a preventable comorbidity of cancer. A TARGET approach. Crit rev oncol hematol 2015;94:251-9
- Argilés JM, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754-62
- Mondello P, et al. Emerging markers of cachexia predict survival in cancer patients. BMC Cancer 2014;14:828
- Onesti JK, Guttridge DC. Inflammation based regulation of cancer cachexia. BioMed Res Int 2014;168407
- Bennani-Baiti N, Walsh D. What is cancer anorexia-cachexia syndrome? A historical perspective. J R Coll Phys Edinb 2009;39:257-62
- Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle 2013;4:95-109
- Dalal S, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manag 2012;44:181-91
- Peng P, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86
- Harimoto N, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg 2013;100:1523-30
- Fearon K, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95
- Grivaux M, et al. Early mortality in lung cancer: French prospective multicentre observational study. BMC Pulm Med 2016;16:45
- Arthur ST, et al. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context 2014;3:212265
- Fox KM, et al. Estimation of cachexia among cancer patients based on four definitions. J Oncol 2009 (doi:10.1155/2009/693458)
- Wallengren O, Lundholm K, Bosaeus I. Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients. Support Care Cancer 2013;21:1569-77
- Ceelen JJ, Langen RC, Schols AM. Systemic inflammation in chronic obstructive pulmonary disease and lung cancer: common driver of pulmonary cachexia? Curr Opin Support Palliat Care 2014;8:339-45
- Baracos VE. Pitfalls in defining and quantifying cachexia. J Cachexia Sarcopenia Muscle 2011;2:71-3
- Pressoir M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer 2010;102:966-71
- Kimura M, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 2014;1-10
- Golinvaux NS, et al. Limitations of administrative databases in spine research: a study in obesity. Spine J 2014;14:2923-8
- Woo JG, et al. Obesity identified by discharge ICD-9 codes underestimates the true prevalence of obesity in hospitalized children. J Pediatrics 2009;154:327-31