Abstract
Objective: To estimate per-event cost and economic burden associated with managing the most common and/or severe metastatic melanoma (MM) treatment-related adverse events (AEs) in Australia, France, Germany, Italy, and the UK.
Methods: AEs associated with chemotherapy (dacarbazine, paclitaxel, fotemustine), immunotherapy (ipilimumab), and targeted therapy (vemurafenib) were identified by literature review. Medical resource use data associated with managing AEs were collected through two blinded Delphi panel cycles in each of the five countries. Published costs were used to estimate per-event costs and combined with AEs incidence, treatment usage, and MM prevalence to estimate the economic burden for each country.
Results: The costliest AEs were grade 3/4 events due to immunotherapy (Australia/France: colitis; UK: diarrhea) and chemotherapy (Germany/Italy: neutropenia/leukopenia). Treatment of AEs specific to chemotherapy (Australia/Germany/Italy/France: neutropenia/leukopenia) and targeted therapy (UK: squamous cell carcinoma) contributed heavily to country-specific economic burden.
Limitations: Economic burden was estimated assuming that each patient experienced an AE only once. In addition, the context of settings was heterogeneous and the number of Delphi panel experts was limited.
Conclusions: Management costs for MM treatment-associated AEs can be substantial. Results could be incorporated in economic models that support reimbursement dossiers. With the availability of newer treatments, establishment of a baseline measure of the economic burden of AEs will be crucial for assessing their impact on patients and regional healthcare systems.
Introduction
Metastatic melanoma is one of the most aggressive forms of cancer. Until recently, cytotoxic chemotherapies, such as dacarbazine, fotemustine, and paclitaxel were used to treat patients with metastatic disease. However, response rates were low (6–26%), with median survival rates reported to be 6–10 months and less than 5% of patients surviving for more than 5 yearsCitation1–3.
In the past 5 years, the melanoma treatment landscape has rapidly evolved with the development of novel immune and targeted therapies. In 2011, ipilimumab, a fully humanized monoclonal antibody directed against a cytotoxic T-lymphocyte-associated antigen 4Citation4, and vemurafenib, a selective BRAF inhibitorCitation5, were first approved in the USCitation6,Citation7, then EuropeCitation8,Citation9 and AustraliaCitation10,Citation11, for the treatment of metastatic melanoma. In 2013–2014, dabrafenib, also a selective BRAF inhibitor, and trametinib, a selective MEK inhibitor, were approved in the USCitation12,Citation13, EuropeCitation14,Citation15, and AustraliaCitation16,Citation17, first as monotherapies, then in combination, for patients with BRAF-mutant melanoma detected by a validated test. In 2014, the anti-PD-1 monoclonal antibodies pembrolizumab and nivolumab were approved for the treatment of metastatic melanoma in the USCitation18,Citation19, soon followed by approvals in EuropeCitation20,Citation21 and Australia (pembrolizumab only)Citation22. In 2015, ipilimumab and nivolumab combination therapy was approved for metastatic melanoma in the USCitation19. These recent approvals have significantly improved the outcomes of patients with metastatic melanoma, with response rates now reaching ≥60% and median overall survival reaching >25 months with combination therapiesCitation4,Citation23–26.
The costs associated with treating and managing cancer can be substantial and impose a considerable economic burden on societyCitation27,Citation28. Studies have evaluated the economic burden of treating metastatic melanoma in EuropeCitation29–31, and the USCitation32, but no details have yet been published specific to the costs of managing the adverse events (AEs) associated with drug treatments. As shown in other cancers, the cost associated with treating/managing AEs are an important consideration from a health economic perspective, as they contribute to the overall economic burden of cancer careCitation33–35. AEs associated with cancer treatments can lead to a high utilization of healthcare resources, including direct medical costs (for example, hospitalization, prescribed medication, and healthcare professional consultation), and indirect medical costs (such as productivity loss and caretaker time). Furthermore, as new therapies become available, it may be important to establish a baseline measure of the economic burden of AEs so that policymakers can assess the impact that new agents have on patients and on healthcare systems.
The objective of this study was to estimate the per-event costs and overall economic burden associated with treating and managing the most common and/or severe AEs for chemotherapy and the first approved novel immune (ipilimumab) and targeted (vemurafenib) therapies frequently used for treating patients with metastatic melanoma across Europe (France, Germany, Italy, and the UK) and Australia. Since the newer agents dabrafenib, trametinib, pembrolizumab, and nivolumab were not approved at the time of this study, they were not included.
Methods
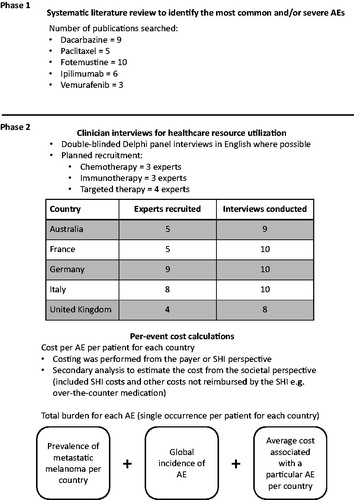
There were two phases to data collection in this project: the first phase involved a systematic review of the literature to identify the AEs to be included in the analysis (); the second phase involved Delphi panel interviews to determine the medical resource use associated with managing these AEs. A cost analysis then followed to estimate the cost per AE per patient and the total population-level burden for each AE per country (assuming one occurrence per patient).
Systematic literature review
A systematic literature review was conducted using standard methodologyCitation36,Citation37 to evaluate the incidence and types of AEs associated with chemotherapy (dacarbazine, paclitaxel, and fotemustine), immunotherapy (ipilimumab), and targeted therapy (vemurafenib) for metastatic melanoma. PubMed was searched for relevant publications published before March 1, 2012. Evaluation of AEs associated with dacarbazine treatment was based on nine studiesCitation5,Citation38–45, five for paclitaxelCitation46–50, 10 for fotemustineCitation51–61, six studies for ipilimumabCitation4,Citation62–66, and three studies for vemurafenib treatmentCitation5,Citation67,Citation68. Dabrafenib and trametinib were not included in the PubMed search, as these treatments were not approved for the treatment of patients with metastatic melanoma at the time of the literature review. Further details and flow chart of literature review are included in the Supplementary Materials.
Delphi interviews
The medical resource use data relating to the specific AEs associated with the different categories of treatment were estimated through clinician interviews using two rounds of double-blinded Delphi panelCitation69,Citation70 interviews in each country. The Delphi panels were double-blinded in terms of the experts not knowing the sponsor and vice versa. All the interviews were planned to be conducted in English. For the purpose maintaining a manageable interview length, the different therapies were combined into three categories: immunotherapy, chemotherapy, and targeted therapy. Due to the length of the questionnaire, the initial intention was to present one category per expert. In Australia, France, Germany, and the UK, some experts responded to questions about ≥1 treatment category where recruitment was difficult. In each country, four experts answered the targeted therapy-related questions, three experts the immunotherapy-related questions, and three experts the chemotherapy-related questions.
After the first Delphi cycle, summarized resource use results were distributed to each panel member for review and comment. A second interview session occurred in order to collect the reflected feedback on the summarized results. Data from the second cycle was again evaluated and summarized. The final analysis was based on these revised data. The interview-based data collection was conducted between August 2012 and May 2013.
Per-patient per-event cost calculations
In summary, the costing was primarily performed from the payer or statutory health insurance (SHI) perspective, which included all the direct costs covered by each country’s SHI system: Medicare (Australia), Sécurité Sociale (France), German statutory health insurance, Servizio Sanitario Nazionale (Italy), and the National Health Service (UK). A secondary analysis to estimate the cost of treating and managing the AEs from the societal perspective (defined as also including out of pocket expenses for patients but not indirect costs associated with productivity loss, caretaker time, etc.) was also performed.
Unit costs collection from literature and public sources (Supplementary Table 1) took place between January and August 2013. Details of medication costs, outpatient services, inpatient care, and cost of ongoing therapies were calculated per country and are described in the Supplementary Materials.
Table 1. Epidemiological and treatment usage data from 5 countries.
When calculating the cost of ongoing therapies, the estimated average time to resolution of AEs was taken into account as provided by the Delphi experts. In case an ongoing duration of therapy was specified, such estimation was capped using calculated average overall survival of 14.4 months for targeted therapiesCitation5,Citation68, 8.2 months for chemotherapyCitation2,Citation5,Citation38–40,Citation44,Citation46,Citation50,Citation54,Citation55,Citation57,Citation58,Citation60,Citation61,Citation71–73, and 12.1 months for immunotherapyCitation4,Citation62–64,Citation74–76.
The costs associated with treating the AEs were calculated as the sum of all the direct costs for a particular AE across all participating experts per country and then as an average cost of the AE per expert in each country. Further details on cost estimation (including breakdowns by resource category) can be found in the Supplementary Materials.
Population-level burden of treating AEs
The burden of treating AEs associated with melanoma therapies was calculated at the population level for each country by combining the average cost associated with a particular AE, by therapy (targeted, chemotherapy, and immunotherapy), the prevalence of metastatic melanoma, treatment usage, and global incidence of the AE (). Our extrapolation of treatment burden is limited to a cost per single occurrence of each AE.
The adult 1-year prevalence of metastatic melanoma in each country was used as a baseline to calculate number of metastatic melanoma patients, from which we estimated the numbers of patients treated with each therapy, as well as each chemotherapy (dacarbazine, fotemustine, or paclitaxel) in the country. We then estimated the number of patients who experienced a particular AE in the country utilizing global incidences of AEs (estimated from the aggregated data already obtained through the literature review; Supplementary Table 2).
Table 2. List of the most common (>20% any grade or >5% grade 3 or higher) adverse events associated with chemotherapy (dacarbazine, paclitaxel and fotemustine), immunotherapy (ipilimumab), and targeted therapy (vemurafenib).
Results
Most common and/or severe AEs
The incidence of AEs associated with chemotherapy, immunotherapy, and targeted treatment are shown in Supplementary Table 2. A total of 29 AEs (11 chemotherapy, seven immunotherapy, 11 targeted therapy) met the pre-defined criteria for inclusion in the subsequent data collection phases (occurred in >20% of patients, irrespective of severity, or in >5% of patients ≥ grade 3; ). This criteria was used because several clinical trial papers report AEs using these cut-offs (i.e. ≥20% of all grade AEs, 5–10% of ≥ grade 3 AEs).
Medical resource information
Results for each treatment category from the Delphi panel showed that in Australia and Italy all patients who required AE management by inpatient hospitalization, or a prolonged stay in hospital, were reported to have grade 3 or 4 AEs (Supplementary Tables 3 and 4). All patients with grade 1 or 2 AEs were managed through outpatient visits. In France, Germany, and the UK, grade 3 or 4 AEs commonly, but not always, required management through inpatient hospitalization. In Australia, Italy, and the UK, the only grade 3 or 4 AEs that did not require hospitalization and were managed by ambulatory or outpatient care were peripheral neuropathy due to chemotherapy, and squamous cell carcinoma due to targeted therapy. In Germany, no AEs of grade 3 or 4 severity were managed only by ambulatory or outpatient care (Supplementary Tables 3 and 4).
In all countries, the mean percentage of patients who required inpatient hospitalization, or a prolonged stay in hospital, appeared to be lower for those receiving targeted treatment compared with those who received chemotherapy or immunotherapy. In Australia, Italy, and the UK, the only AE associated with targeted therapy that was managed through inpatient hospitalization, or a prolonged stay in hospital, was grade 3 or 4 rash (Supplementary Tables 3 and 4).
Per-patient per-event cost and population-level burden analysis
Australia
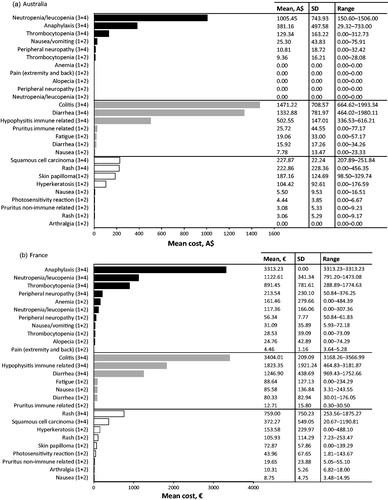
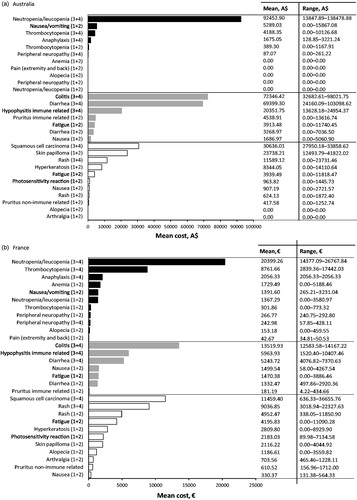
In Australia, the most cost intensive AE to treat from the SHI perspective at the patient level was associated with immunotherapy, specifically grade 3 or 4 colitis (; ). The mean cost of treating this AE was estimated to be A$1471.22 per event per patient (PEPP). The next most cost intensive AE to treat was also associated with immunotherapy, grade 3 or 4 diarrhea, with a mean cost of A$1332.88 PEPP. Both these costs were associated with patients being admitted to the hospital oncology ward to receive intravenous (IV) steroids, anti-diarrheals, or fluids (mean colitis costs A$1399.93 PEPP; mean diarrhea costs A$1272.67 PEPP), followed by the costs incurred due to consultation of a healthcare professional for monitoring and medication prescription (mean colitis costs A$64.06 PEPP; mean diarrhea costs A$50.26 PEPP).
Figure 2. Cost calculation per event per patient, for 29* adverse events (AEs) across 3 therapy modes, from the statutory health insurance (SHI) perspective for (a) Australia, (b) France, (c) Germany, (d) Italy and (e) United Kingdom. Adverse events are grouped together for chemotherapy (black bars), immunotherapy (grey bars) and targeted therapy (white bars); AE grades are denoted in brackets, i.e., grade 1 and 2 are (1+2). *Alopecia and fatigue frequently occur also due to targeted therapy, however the cost for the two AEs is shown only within the chemotherapy and immunotherapy categories. For the two AEs we have assumed that management and the cost would not differ among the therapies.
Table 3. Top 10 most cost intensive adverse events (AE) (based on per event per patient) from the statutory health insurance perspective. Adverse events due to chemotherapy (dark grey), immunotherapy (light grey), and targeted therapy (white) are ranked from most expensive to least expensive. All AEs listed are grade 3 or 4 unless specified.
The third most cost intensive AE to treat in Australia was grade 3 or 4 neutropenia/leukopenia associated with chemotherapy (), with a mean cost of A$1005.45 PEPP. This cost was associated with patients being admitted to the hospital oncology ward to receive IV antibiotics (mean cost A$1004.00 PEPP).
In terms of the population-level burden of managing specific AEs associated with metastatic melanoma treatments, the highest burden in Australia was for the management of grade 3 or 4 neutropenia/leukopenia associated with chemotherapy treatment, followed by grade 3 or 4 colitis, and 3 or 4 diarrhea; both of which were associated with immunotherapy ().
Figure 3. Overall (population-level) burden of treatment of specific adverse events (AEs) per therapy class in (a) Australia, (b) France, (c) Germany, (d) Italy and (e) United Kingdom. Adverse events are grouped together for chemotherapy (black bars), immunotherapy (grey bars) and targeted therapy (white bars); AE grade are denoted in brackets, i.e., grade 1 and 2 are (1+2).
France
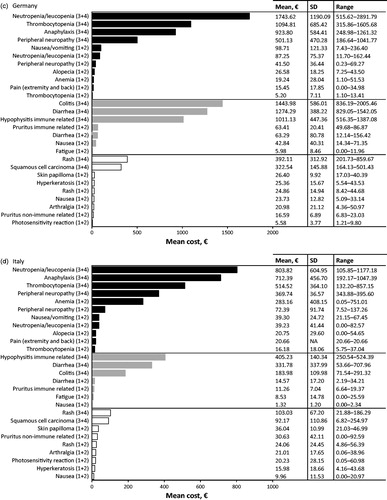
In France, the most cost intensive AE to treat from the SHI perspective at the patient level was grade 3 or 4 colitis associated with immunotherapy (; ). The mean cost of treating this AE was €3404.01 PEPP. Associated costs were due to inpatient hospitalization in dermatology wards to receive medication such as corticosteroids, IV nutrition, fluids, and painkillers (mean cost €3360.90 PEPP), followed by costs of consulting a healthcare professional (mean cost €35.26 PEPP).
The second most cost intensive AE to treat was associated with grade 3 or 4 anaphylaxis due to chemotherapy (; ), with a mean cost of €3313.23 PEPP. The highest cost associated with anaphylaxis was due to inpatient hospitalizations to dermatology or dermato-oncology wards to receive medications (e.g. systemic corticosteroids and/or adrenaline; mean cost €3313.23 PEPP).
The third most cost intensive AE to treat was associated with immunotherapy, grade 3 or 4 immune-related hypophysitis (), with a mean cost of €1823.35 PEPP. This cost was associated with patients being admitted to oncology, dermatology, or gastroenterology wards for rehydration, corticosteroid therapy, IV nutrition, and diagnostic procedures, such as endoscopy and stool culture (mean cost €1675.78 PEPP).
At the population level in France, grade 3 or 4 neutropenia/leukopenia associated with chemotherapy contributed the highest overall management costs. The next highest costs were due to grade 3 or 4 colitis associated with immunotherapy, and grade 3 or 4 squamous cell carcinoma associated with targeted therapy ().
Germany
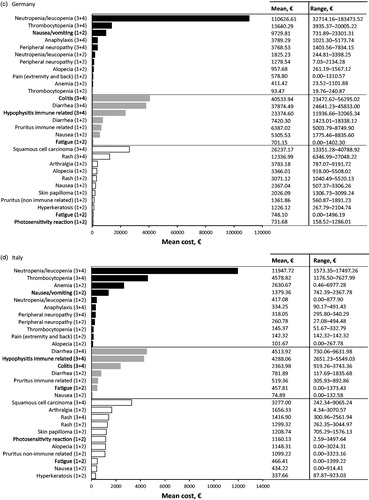
In Germany, the most cost intensive AE to treat from the SHI perspective at the patient level was associated with chemotherapy, grade 3 or 4 neutropenia/leukopenia (; ), with a mean cost of €1743.62 PEPP. The highest contributing costs here were prescribed medication (e.g. pegfilgrastim; mean cost €927.86 PEPP), followed by consultation and advice from the internal medicine department of a hospital (mean cost €767.50 PEPP).
The second and third most cost intensive AEs were due to immunotherapy (): grade 3 or 4 colitis (mean cost €1443.98 PEPP), and grade 3 or 4 diarrhea (mean cost €1274.29 PEPP). The highest contributing cost to colitis was inpatient hospitalization to gastroenterology or internal medicine ward for medication, diagnostic procedures or supportive care (mean cost €1322.67 PEPP). The highest contributing cost to diarrhea was associated with patients being admitted to dermato-oncology, or internal medicine wards for medication or diagnostic procedures (mean cost €1167.21 PEPP).
At the population level in Germany, grade 3 or 4 neutropenia/leukopenia associated with chemotherapy treatment contributed the highest overall management costs. The next highest costs were due to grade 3 or 4 colitis, and grade 3 or 4 diarrhea, both associated with immunotherapy ().
Italy
In Italy, the top three most cost intensive AEs to treat from the SHI perspective at the patient level were all associated with chemotherapy: grade 3 or 4 neutropenia/leukopenia, grade 3 or 4 anaphylaxis and grade 3 or 4 thrombocytopenia (; ). The mean costs of treating these AEs were €803.82, €712.39, and €514.52 PEPP, respectively.
The high costs associated with neutropenia/leukopenia were due to medications, such as pegfilgrastim (mean cost €561.27 PEPP), followed by inpatient hospitalizations to oncology ward for medications such as IV antibiotics and granulocyte stimulating factor (mean cost €181.76 PEPP). For anaphylaxis, the high costs were associated with inpatient hospitalization to the oncology ward (mean cost €544.07 PEPP), prescribed medication (mean cost €151.09 PEPP) and oxygen (mean cost €452.10 PEPP) being the most expensive.
At the population level in Italy, the highest AE management costs were contributed by grade 3 or 4 neutropenia/leukopenia, followed by grade 3 or 4 thrombocytopenia; both were associated with chemotherapy treatment. The next highest costs were due to grade 3 or 4 diarrhea associated with immunotherapy ().
United Kingdom
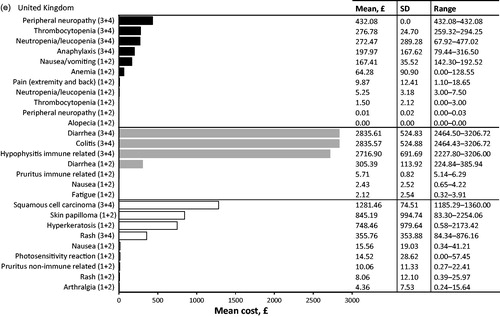
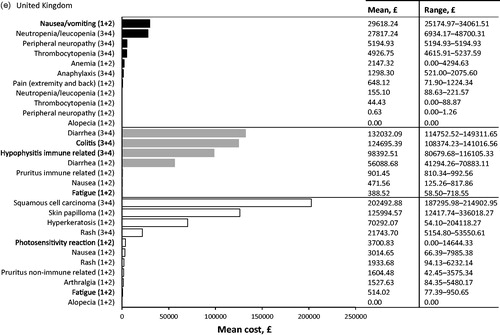
In the UK, the three most cost intensive AEs to treat from the SHI perspective at a patient level were associated with immunotherapy: grade 3 or 4 diarrhea, grade 3 or 4 colitis and grade 3 or 4 immune-related hypophysitis (; ). The mean cost of treating diarrhea was £2835.61 PEPP, for colitis £2835.57 PEPP, and for immune-related hypophysitis £2716.90 PEPP. The highest contributing costs associated with the treatment of diarrhea or colitis was inpatient hospitalization to the oncology ward to receive IV steroids or an endoscopy (mean diarrhea costs £2772.00 PEPP; mean colitis costs £2772.00 PEPP). The high costs associated with treatment of immune-related hypophysitis were due to inpatient hospitalization to the oncology ward to receive IV steroids (mean cost £2618.00 PEPP).
In terms of the specific population level burden of managing AEs associated with treating metastatic melanoma in the UK, the highest costs were for management of grade 3 or 4 squamous cell carcinoma associated with targeted therapy. The next highest costs were due to grade 3 or 4 diarrhea associated with immunotherapy followed by grade 1 or 2 skin papilloma due to targeted treatment ().
The most cost intensive AEs
reports the top 10 most expensive AEs (per patient per event) from a SHI perspective for each of the five countries. The majority of cost intensive AEs (ranked 1–3) across the three treatment categories were associated with either chemotherapy or immunotherapy and were grade 3 or 4 in severity.
Discussion
Until recently, treatment options for patients with metastatic melanoma were limited. Treatments such as ipilimumab and vemurafenib, both approved in 2011, have significantly improved patient outcomes in the past 5 years; however, as the melanoma treatment landscape continues to evolve, the costs associated with treating and managing melanoma may impose a considerable economic burden on society. This is the first study to assess costs associated with the management of AEs specifically attributable to treatments for patients with metastatic melanoma in Europe and Australia.
Results from the Delphi panel highlighted the differences across the five countries in the way AEs are managed (see Supplementary Tables 3 and 4). The per-event cost analysis demonstrated that the costs of managing AEs associated with chemotherapy, immunotherapy, and targeted therapy can be substantial in all countries. Grade 3/4 AEs were the most costly to treat across all three treatment categories (), with hospitalization and prescribed medication being the main underlying drivers of this cost.
Consistent with the average per-patient cost per AE, grade 3 or 4 AEs resulted in a very high population level cost burden in each country. Chemotherapy was associated with the highest population level burden in Australia, Germany, Italy, and France (mainly due to neutropenia/leukopenia), whilst in the UK it was targeted therapy (due to the cost of treatment of squamous cell carcinoma). There are some shortcomings in making comparisons across countries due to potential differences in reimbursement status, physician’s choice of treatment, and patient’s disease characteristics. The incidences of AEs were also derived from studies of different durations (5–74 months; average 29.4 months)Citation4,Citation5,Citation38–46,Citation48,Citation50,Citation53,Citation54,Citation56,Citation58–60,Citation62,Citation64,Citation68,Citation74, and we assumed that each patient experienced an AE only once in the course of their treatment, due to use of limited data on recurrence of AEs. Further, the use of Delphi panels from a limited number of centers may not fully represent the usage in clinical settings countrywide and could introduce some bias to the usage estimates. The total burden of AE treatment is also likely to be an under-estimate given that (i) multiple AE occurrences were not accounted for, (ii) the prevalence estimate was from 2008, (iii) the melanoma incidence is risingCitation77, and (iv) in the case of Germany and France the results focus on patients diagnosed at a metastatic stage, excluding patients diagnosed at an early stage who will progress to metastatic stage within a year.
The results of this study suggest that the per-patient cost of treating AEs can be as high as thousands of Euros. Such figures may be insignificant compared with the high cost of the newer melanoma medications, but, nevertheless, the quantification of the cost of AE treatment will prove useful in the overall disease burden discussions, economic modelling, and budget considerations for healthcare providers. Newer targeted therapies and immunotherapies alone or in combination are likely to be associated with a similar set of AEs, for which this study will provide a source of data about potential cost burden.
The BRAF inhibitor dabrafenib has a similar safety profile to vemurafenib, but is generally associated with fewer cutaneous adverse events including SCC and rash and a higher incidence of pyrexia, an AE uncommon in patients receiving vemurafenib therapyCitation24,Citation78–81. Combination of BRAF inhibitors with MEK inhibitors including dabrafenib plus trametinib and vemurafenib plus cobimetinib typically demonstrate reduced cutaneous toxicity including SCC and rash compared with BRAF inhibitor monotherapy, but higher rates of pyrexia in the case of dabrafenib and trametinib and central serous retinopathy and photosensitivity with vemurafenib plus cobimetinibCitation24,Citation82. Similar to ipilimumab, the most notable AEs with anti-PD-1 monoclonal antibodies nivolumab and pembrolizumab are typically immune-related, but anti-PD-1 therapy appears to be associated with fewer incidence of colitis and a potential increase in thyroid abnormalitiesCitation23,Citation83. The safety profile of combination CTLA-4 and PD-1 blockade is similar to the observed profile for each as monotherapy, but with greater frequencyCitation23. Future analysis may be warranted to understand the economic burden of differential safety profiles of newer agents and combinations as their use expands in clinical settings.
Studies evaluating the costs of AEs for particular treatments have been carried out in other cancersCitation33–35, but, to our knowledge, no other studies have evaluated the cost of AEs relating to specific therapies that have been used to treat patients with metastatic melanoma in Europe or Australia. A similar study from the US healthcare perspective evaluated the incremental healthcare costs of specific AEs among patients receiving existing and new therapies for the treatment of metastatic melanoma, and found that incremental costs associated with specific treatment-related AEs were substantialCitation84. The studies in other cancers showed the importance of evaluating the AEs associated with particular treatments and the economic burden on cancer services. They also highlighted that efforts towards preventing these AEs may be important for reducing the financial burden in oncologyCitation34,Citation35. As part of the Italian component of a European observational study, MELODY, the cost of AE management for patients with unresectable stage III/IV melanoma (transfusion, administration of concomitant medications including antiemetics, and growth factors) receiving the most commonly prescribed agents has been evaluated. It concluded that the most expensive category of medication was immunostimulants (€785–€3051 per episode)Citation30.
Conclusions
In conclusion, this study is the first to provide an estimate of the cost burden associated with managing the most common and/or severe AEs associated with chemotherapy, ipilimumab, and vemurafenib. The costs associated with management of AEs associated with chemotherapy, immunotherapy, and targeted treatments for metastatic melanoma are substantial. As recently-approved novel treatments become more widely available, establishment of a baseline measure of the economic burden of AEs will be crucial for assessing the impact of new agents on patients and regional healthcare systems.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline.
Declaration of financial/other relationships
MA, AM, CS, and SP were employees of GlaxoSmithKline during conduction of the study and initial publication development. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental material
Download MS Word (956.5 KB)Acknowledgments
Editorial support in the form of development of draft outline and first draft, editorial suggestions to draft versions of the paper, collating author comments, references, and graphic services was provided by SciMentum and ArticulateScience, LLC funded by GlaxoSmithKline and Novartis. The authors would like to acknowledge the Delphi panel experts for their assistance in data provision and analysis and Dr Bertrand Téhard (PhD, HEOR Project Manager at GlaxoSmithKline at the time of study) for his assistance in conducting the economic valorisation of hospital costs for France with Cemka-Eval.
References
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med 2004;351:998-1012
- Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol 2004;22:1118-25
- Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488-96
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16
- Genentech. Zelboraf US prescribing information. 2011. South San Francisco, CA, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202429s000lbl.pdf. Accessed October 6, 2015
- Bristol-Myers Squibb. Yervoy (ipilimumab) [package insert]. 2013. Princeton, NJ, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125377s055lbl.pdf. Accessed October 6, 2015
- Genentech. Zelboraf EMA summary of product characteristics. 2012. Grenzach-Wyhlen, Germany. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002409/WC500124317.pdf. Accessed October 6, 2015
- Bristol-Myers Squibb. Yervoy EMA summary of product characteristics. 2011. East Syracuse, NY. Grenzach-Wyhlen, Germany. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002213/WC500109299.pdf. Accessed October 6, 2015
- Roche. Zelboraf AU product information. 2015. Dee Why NSW, Australia http://www.guildlink.com.au/gc/ws/ro/pi.cfm?product=ropzelbo10615. Accessed May 9, 2016
- Bristol-Myers Squibb. Yervoy AU product information. 2015. Victoria, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2011-PI-02907-3. Accessed May 9, 2016
- Novartis Pharmaceuticals Corporation. Tafinlar US prescribing information. 2014. East Hanover, NJ, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202806s002lbl.pdf. Accessed October 6, 2015
- Novartis Pharmaceuticals Corporation. Mekinist US prescribing information. 2014. East Hanover, NJ, USA. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204114s001lbl.pdf. Accessed January 10, 2014
- Novartis Pharmaceuticals Corporation. Tafinlar EMA summary of product characteristics. 2015. Camberley, Surrey, UK. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002604/WC500149671.pdf. Accessed October 12, 2015
- Novartis Pharmaceuticals Corporation. Mekinist EMA product characteristics. 2015. Camberley, Surrey, UK. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002643/WC500169657.pdf. Accessed October 12, 2015
- Novartis Pharmaceuticals Corporation. Tafinlar AU product information. 2015. Macquarie Park, NSW, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2013-PI-02126-1. Accessed May 9, 2016
- Novartis Pharmaceuticals Corporation. Mekinist AU product information. 2015. Macquarie Park, NSW, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2014-PI-01394-1. Accessed May 6, 2016
- Merck Sharp & Dohme. Keytruda US prescribing information. 2015. County Carlow, Ireland. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed October 12, 2015
- Bristol-Myers Squibb Company. Opdivo US prescribing information. 2015. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed May 9, 2016
- Merck Sharp & Dohme. Keytruda EMA product information. 2015. Frederick, MD, USA. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003820/WC500190990.pdf. Accessed October 12, 2015
- Bristol-Myers Squibb. Opdivo EMA product characteristics. 2015. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Princeton, NJ, USA. Accessed October 12, 2015
- Merck Sharp & Dohme. Keytruda AU product information. 2015. Macquarie Park, NSW, Australia. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2015-PI-01639-1. Accessed May 9, 2016
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23-34
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015;386:444-51
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-19
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9
- Luengo-Fernandez R, Leal J, Gray A, et al. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol 2013;14:1165-74
- Commission of the European Communities. Communication from the commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on action against cancer: European partnership. Brussels, Belgium. 2009. http://ec.europa.eu/health/ph_information/dissemination/diseases/docs/com_2009_291.en.pdf. Accessed May 20, 2016
- Johnston K, Levy AR, Lorigan P, et al. Economic impact of healthcare resource utilisation patterns among patients diagnosed with advanced melanoma in the United Kingdom, Italy, and France: results from a retrospective, longitudinal survey (MELODY study). Eur J Cancer 2012;48:2175-82
- Maio M, Ascierto P, Testori A, et al. The cost of unresectable stage III or stage IV melanoma in Italy. J Exp Clin Cancer Res 2012;31:91
- Lorigan P, Marples M, Harries M, et al. Treatment patterns, outcomes, and resource utilization of patients with metastatic melanoma in the U.K.: The MELODY study. Br J Dermatol 2014;170:87-95
- Ekwueme DU, Guy GP, Jr., Li C, et al. The health burden and economic costs of cutaneous melanoma mortality by race/ethnicity-United States, 2000 to 2006. J Am Acad Dermatol 2011;65(5 Suppl 1):S133-S43
- Banz K, Bischoff H, Brunner M, et al. Comparison of treatment costs of grade 3/4 adverse events associated with erlotinib or pemetrexed maintenance therapy for patients with advanced non-small-cell lung cancer (NSCLC) in Germany, France, Italy, and Spain. Lung Cancer 2011;74:529-34
- Borovicka JH, Calahan C, Gandhi M, et al. Economic burden of dermatologic adverse events induced by molecularly targeted cancer agents. Arch Dermatol 2011;147:1403-9
- Mickisch G, Gore M, Escudier B, et al. Costs of managing adverse events in the treatment of first-line metastatic renal cell carcinoma: bevacizumab in combination with interferon-alpha2a compared with sunitinib. Br J Cancer 2010;102:80-6
- Khan KS, Kunz R, Kleijnen J, et al. Five steps to conducting a systematic review. J R Soc Med 2003;96:118-21
- Davies HTO, Crombie IK. What is a systematic review? Hayward Medical Communications, a division of Hayward Group plc. West Malling, Kent, UK. 2009. http://vivrolfe.com/ProfDoc/Assets/Davis%20What%20is%20a%20systematic%20review.pdf. Accessed May 9, 2016
- Bedikian AY, DeConti RC, Conry R, et al. Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol 2011;22:787-93
- O'Day S, Pavlick A, Loquai C, et al. A randomised, phase II study of intetumumab, an anti-alphav-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer 2011;105:346-52
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26
- Costanza ME, Nathanson L, Costello WG, et al. Results of a randomized study comparing DTIC with TIC mustard in malignant melanoma. Cancer 1976;37:1654-9
- Carter RD, Krementz ET, Hill GJ 2nd, et al. DTIC (nsc-45388) and combination therapy for melanoma. I. studies with DTIC, BCNU (NSC-409962), CCNU (NSC-79037), vincristine (NSC-67574), and hydroxyurea (NSC-32065). Cancer Treat Rep 1976;60:601-9
- Hill GJ 2nd, Ruess R, Berris R, et al. Chemotherapy of malignant melanoma with dimethyl traizeno imidazole carboxamide (DITC) and nitrosourea derivatives (BCNU, CCNU). Ann Surg 1974;180:167-74
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66
- Johnson RO, Metter G, Wilson W, et al. Phase I evaluation of DTIC (NSC-45388) and other studies in malignant melanoma in the central oncology group. Cancer Treat Rep 1976;60:183-7
- O'Day S, Gonzalez R, Lawson D, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol 2009;27:5452-8
- Legha SS, Ring S, Papadopoulos N, et al. A phase II trial of taxol in metastatic melanoma. Cancer 1990;65:2478-81
- Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs 1991;9:59-64
- Wiernik PH, Schwartz EL, Einzig A, et al. Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol 1987;5:1232-9
- Walker L, Schalch H, King DM, et al. Phase II trial of weekly paclitaxel in patients with advanced melanoma. Melanoma Res 2005;15:453-9
- Calabresi F, Aapro M, Becquart D, et al. Multicenter phase II trial of the single agent fotemustine in patients with advanced malignant melanoma. Ann Oncol 1991;2:377-8
- Khayat D, Bizzari JP, Frenay M, et al. Interim report of phase II study of new nitrosourea S 10036 in disseminated malignant melanoma. J Natl Cancer Inst 1988;80:1407-8
- Kleeberg UR, Engel E, Israels P, et al. Palliative therapy of melanoma patients with fotemustine. Inverse relationship between tumour load and treatment effectiveness. A multicentre phase II trial of the EORTC-Melanoma Cooperative Group (MCG). Melanoma Res 1995;5:195-200
- Siegel R, Hauschild A, Kettelhack C, et al. Hepatic arterial fotemustine chemotherapy in patients with liver metastases from cutaneous melanoma is as effective as in ocular melanoma. Eur J Surg Oncol 2007;33:627-32
- Falkson CI, Falkson G, Falkson HC. Phase II trial of fotemustine in patients with metastatic malignant melanoma. Invest New Drugs 1994;12:251-4
- Egerer G, Lehnert T, Max R, et al. Pilot study of hepatic intraarterial fotemustine chemotherapy for liver metastases from uveal melanoma: a single-center experience with seven patients. Int J Clin Oncol 2001;6:25-8
- Schallreuter KU, Wenzel E, Brassow FW, et al. Positive phase II study in the treatment of advanced malignant melanoma with fotemustine. Cancer Chemother Pharmacol 1991;29:85-7
- Leyvraz S, Spataro V, Bauer J, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol 1997;15:2589-95
- Khayat D, Cour V, Bizzari JP, et al. Fotemustine (S 10036) in the intra-arterial treatment of liver metastasis from malignant melanoma. A phase II study. Am J Clin Oncol 1991;14:400-4
- Mornex F, Thomas L, Mohr P, et al. A prospective randomized multicentre phase III trial of fotemustine plus whole brain irradiation versus fotemustine alone in cerebral metastases of malignant melanoma. Melanoma Res 2003;13:97-103
- Mornex F, Thomas L, Mohr P, et al. Randomised phase III trial of fotemustine versus fotemustine plus whole brain irradiation in cerebral metastases of melanoma. Cancer Radiother 2003;7:1-8
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155-64
- Di Giacomo AM, Danielli R, Calabro L, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother 2011;60:467-77
- Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res 2007;13:6681-8
- Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg 2011;27:e87-8
- Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 2011;164:303-7
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809-19
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14
- Dalkey NC, Helmer O. An experimental application of the Delphi method to the use of experts. Manage Sci 1963;9:458-67
- Pill J. The Delphi method: substance, context, a critique and the annotated bibliography. Socioecon Planning Sci 1971;5:57-71
- Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006;17:563-70
- Patel PM, Suciu S, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur J Cancer 2011;47:1476-83
- Cui CL, Chi ZH, Yuan XQ, et al. Hepatic intra-arterial bio-chemotherapy for the treatment of melanoma patients with liver metastasis: a phase II clinical study. Ai Zheng 2008;27:845-50
- Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010;116:1767-75
- Hersh EM, O'Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs 2011;29:489-98
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591-8
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95
- Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205-11
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521-32
- Arondekar B, Curkendall S, Monberg M, et al. Economic burden associated with adverse events in patients with metastatic melanoma. J Manag Care Spec Pharm 2015;21:158-64
- Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012;380:1840-50
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-17
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133-45
- Soerjomataram I, Lortet-Tieulent J, Parkin DM, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012;380:1840-50
- Oncosight. Oncosight patient populations 2010-2015, plan A, appendix 1. Stanford, CA, USA. 2010
- Kassir N, Mouksassi M, Cox DS, et al. Population pharmacokinetics (PK) of trametinib (GSK1120212), a MEK inhibitor, in subjects with cancer. Clin Pharmacol Ther 2013;93(1 Suppl):S69
- Garbe C, Blum A. Epidemiology of cutaneous melanoma in Germany and worldwide. Skin Pharmacol Appl Skin Physiol 2001;14:280-90
- Lacy KE, Karagiannis SN, Nestle FO. Advances in the treatment of melanoma. Clin Med 2012;12:168-71