Abstract
Background: Trastuzumab was considered a cost-effective adjuvant treatment for HER 2-positive early breast cancer. Since 2010, the Taiwanese National Health Insurance (NHI) has started to reimburse for 1-year adjuvant treatment. This study aims to provide an updated cost-effectiveness analysis from the NHI perspective, which explores assumptions about long-term cardiac toxicity and treatment benefit of 1-year adjuvant treatment sequentially after chemotherapy.
Methods: A Markov model was used to evaluate the cost-effectiveness of 1-year adjuvant trastuzumab for HER-2/neu positive early breast cancer over a 20-year life-time horizon. A probability sensitivity analysis using Monte Carlo simulation was performed to characterize uncertainties in the expected outcomes, which are expressed as an incremental costs effectiveness ratio (ICER, cost/QALY). A willingness-to-pay threshold of 3-times the per capita gross domestic product was adopted according to the WHO definition. The Taiwan per capita gross domestic product in 2015 was US$22,355; thus, a threshold was considered as NT$2,011,950 (US$67 065, 1USD =30 NTD in 2015).
Results: The model showed that adjuvant trastuzumab treatment in HER-2/neu positive early breast cancer yielded 1.631 quality-adjusted life-years (QALY) compared with no trastuzumab treatment. The ICER was US $51,863 per QALY gained in the base-case scenario. The Monte Carlo simulation by varying all variables simultaneously demonstrated that the probability of cost-effectiveness at the willingness-to-pay threshold of US$67,065 was 50% for 1-year adjuvant trastuzumab.
Conclusions: From this real-world study, 1-year adjuvant trastuzumab treatment is likely to be a cost-effective therapy for patients with HER-2 positive breast cancer at the willingness-to-pay threshold of 3-times GDP per capita in Taiwan.
Introduction
Breast cancer is the fourth leading cause of cancer death in TaiwanCitation1. According to the registry data available in 2012, the standardized incidence rate of female breast cancer was 10,056 and 10,525 per 100,000 females in 2011 and 2012, respectivelyCitation2. The Taiwanese mortality rate was ranked at 31 (10.5%) compared to the 35 OECD countries, and there has been an uptrend in morbidity and mortality over the past several years. Interestingly, the median age at first diagnosis of female breast cancer has remained at 45–49 years in Asian countries such as Taiwan, China, South Korea, and Japan during the past 2 decades—at least 10 years younger than women in Western countriesCitation3–8. Therefore, preventing and reducing breast cancer deaths is one of the most important issues for the Health Promotion Administration in Taiwan.
Trastuzumab use as adjuvant treatment for early breast cancer that over-expresses Human Epidermal growth factor Receptor 2 (HER2) is well establishedCitation9–11; however, trastuzumab-related cardiotoxicity has been raising oncologist and cardiologist concerns, although it may reverse after a withdrawal from treatmentCitation12. Patients with cardiotoxicity may have a worse prognosis and an important impact on average life expectancy and consequent cost-effectiveness, but only a few studies reported data regarding the association of cardiotoxicity with trastuzumab outside clinical-trial settings. Until recently, there have been studies confirming that trastuzumab may associate with cardiotoxicity in treated women, particularly in the elderlyCitation13,Citation14. Therefore, an updated cost-effectiveness analysis (CEA) including cardiotoxicity-incurred treatment cost is needed. There is no CEA study based on actual claim data since the approval of reimbursement for 1-year adjuvant trastuzumab therapy for early breast cancer in Taiwan. We intended to perform this study assessing whether long-term reimbursement of adjuvant trastuzumab for 1 year is cost-effective from the perspective of the Taiwan Health Insurance Bureau.
Methods
Markov model
A Markov model was used to assess the cost-effectiveness of 1-year adjuvant trastuzumab treatment for women with HER2-positive early breast cancer. The model incorporated five key health states identified in the progression of breast cancer (1) disease-free state (DFS), defined as the health state after surgery and chemotherapy; (2) local recurrence; (3) cardiac events; (4) metastasis; and (5) deathCitation15. The model was simulated from the National Health Insurance (NHI) perspective. The starting age of the patient cohort was 50 years, matching the majority of clinical trials for HER2-positive early breast cancer and actual dataCitation9–11. A patient in the model was considered to be in one of five health states at any time. All patients entered the model at the initiation of trastuzumab therapy (or at the disease-free state in the non-trastuzumab group) and transitioned from one state to another on the basis of the transition probabilities. The cycle length was 1 year and the time horizon was the 20-year lifetime. We used the definition of willingness-to-pay (WTP) threshold suggested by the WHO: 3-times the per capita gross domestic product (GDP)Citation15,Citation16. The Taiwan per capita gross domestic product in 2015 was US$22,355; thus a WTP threshold was considered as US$67 065 (NTD 2,011,950; 1 USD = NT$30 in 2015). The transition probabilities and quality-of-life using utility values assigned to each state were derived from available published literatureCitation17–23. Results are presented as incremental cost-effectiveness ratios (ICERs) in cost per quality-adjusted life year (QALY) gained. All costs and health outcomes were discounted at 3% to adjust for the relative value of the Taiwan dollar at present. The schematic of the Markov model is shown in . Model parameters are described in .
Figure 1. Markov model. Arrows indicate transitions. Arrows that curve back to the same state represent remains in the same state. DFS: Disease free survival. CHF: congestive heart failure.
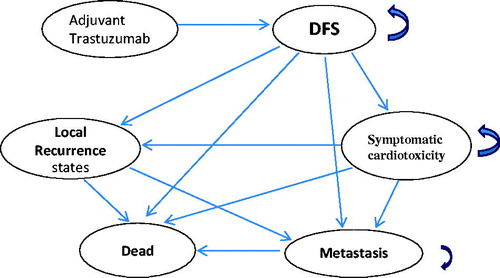
Table 1. Parameters data in sensitivity analysis.
Treatment regimen
Two treatment scenarios were examined from the data retrieved from the NHI database. The first, a trastuzumab group, defined by the reimbursement criteria that trastuzumab is used as an adjuvant treatment only reimbursed for 1 year pre- or post-operation or after standard chemotherapy, and a second non-trastuzumab group defined as the commonly used chemotherapy regimen for HER-2+ early breast cancer women without trastuzumab (such as combination of decetaxel or paclitaxel, doxorubicin and cyclophosphamide, which were identified by ATC code and ICD-9 code 174.9). The initiate dose of trastuzumab sequentially administered within 3 weeks after chemotherapy was 8 mg/kg IV infusion over 90 min. The dose of subsequent therapy was 6 mg/kg IV infusion over 30–90 min every 3 weeks for a total of 17 doses (52 weeks of therapy).
Costs
All direct medical costs for each patient, including physician visits, pharmacist-dispensing fees, laboratory and diagnostic tests as well as additional treatments (surgery, hormonal therapy, chemotherapy, radiotherapy and special interventions) were retrieved directly from the NHI database. The average direct medical cost for each health state was then calculated and used to estimate the annual medical expenditures under different health states. Costs associated with detection and treatment of cardiac events were also retrieved from the database.
Sensitivity analysis
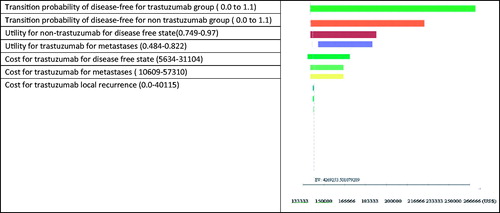
A one-way sensitivity analysis was conducted to determine the potential impact of different parameters on this analysis; the result was presented as a tornado diagram. We hypothesized that the parameters varied over a range of minimum and maximum values (), which was retrieved from the published literature and approved by the experts. A probability sensitivity analysis (PSA) using a Monte Carlo simulation was performed to assess the impact of uncertainty around the key parameters of the model on the incremental cost-effectiveness ratio (ICER). That is, distributions for each parameter with the probabilistic sensitivity analysis were modeled. Log-normal distributions were adopted for all costs and beta distributions were adopted for probabilities, utilities, and toxicity. The probabilistic sensitivity analysis was based on 10,000 samples, and the results are presented as a cost-effectiveness acceptability curve.
Results
Base-case analysis
The direct medical costs in different health states were calculated by the total direct medical cost retrieved from the database as the criteria for patients with HER-2 positive early breast cancer who received treatment regimen with trastuzumab or without trastuzumab. The first year of medical costs, including cost of surgery, chemotherapy, toxicity, and trastuzumab, in the DFS was higher than that of the subsequent year. Adjuvant trastuzumab treatment yielded an ICER of USD $51 863 (NTD 1,711,485) per QALY gained in the base-case scenario (). Based on the WTP threshold as defined in Taiwan, 1-year adjuvant trastuzumab for breast cancer was a cost-effective therapy.
Table 2. Cost-effectiveness analysis for lifetime model.
Sensitivity analysis
One-way sensitivity analyses showed important parameters driving the model. The tornado analysis showed that the key parameters of the model were transition probabilities of the DFS for both the trastuzumab and non-trastuzumab group; health utility for non-trastuzumab group in disease free state; utility for trastuzumab in metastasis state ().
Figure 3. (a) Incremental cost-effectiveness plane. A sample of 10,000 results of the Markov Chain Monte Carlo simulation are plotted. The diagonal line represents a willingness-to-pay threshold of this study, where estimates below this line are cost-effective. (b) Cost-effectiveness acceptability curve. These show that probability that a specific treatment is cost-effective at a given willingness-to-pay threshold of US$67,060 (NT$2,213,145).
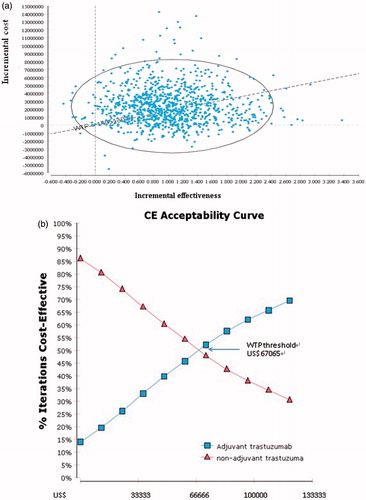
The Monte Carlo simulation by varying all variables simultaneously demonstrated that the probability of cost-effectiveness at the WTP threshold of US$67 065 per QALY gained was 50% chance for 1-year adjuvant trastuzumab (). The ICER range is shown in .
Discussion
Our analysis indicates that 1 year of adjuvant trastuzumab after surgery or chemotherapy is a cost-effective treatment option compared with chemotherapy alone. The incremental cost per QALY gained was estimated to USD 51 863 (NTD 1 711 485) for women with HER2-positive early breast cancer. These estimates of the cost per QALY gained are 50% chance at the WTP threshold of US$67 065 used in Taiwan (no consensus WTP threshold has yet been determined so we, therefore, used the WHO cost-effectiveness threshold). To our knowledge, this is a first study in Taiwan to assess the cost-effectiveness of 1-year adjuvant trastuzumab after surgery or chemotherapy for HER +2 early breast cancer after the reimbursement by the national healthcare plan. Therefore, the result of this study could present valuable information for decision-markers to re-evaluate their payment policy on whether it is still of value to pay for the 1-year adjuvant treatment strategy continuously.
Adjuvant trastuzumab has previously been proven to be a cost-effective treatment for HER2-positive early breast cancer in many countries. Most of these studies were performed before 2009 and did not include the treatment cost of grade 3 or 4 cardiotoxicityCitation23–25. Therefore, the ICER may be under-estimated for the lifetime model; however, few studies performed beyond 2009 including treatment cost of cardiotoxicity reported that 1-year adjuvant trastuzumab treatment is not cost-effective using the definition of WHO cost-effectiveness threshold of 3-times GDP per capitaCitation26,Citation27. The reason may be due to the high price of drugs and lower per capita GDP in those countries.
The strength of our study was that our cost data was retrieved directly from actual claims data, eliminating the impact of bias from using questionnaire surveys of clinical experts. The limitation of our study was that some clinical oncologist omitted precise ICD-9 of cardiotoxicity in the patients’ medical record. Therefore, the cost of treating cardiotoxicity may be under-estimated because it may not be directly reflected in the claim database.
In conclusion, from this real-world study, 1-year adjuvant trastuzumab treatment is likely to be a cost-effective therapy for patients with HER-2 positive breast cancer at the WTP threshold of 3-times GDP per capita in Taiwan.
Transparency
Declaration of funding
The authors report no funding for this manuscript.
Declaration of financial/other relationships
The authors report no financial/other relationships to declare for this manuscript. JME peer reviewers on this manuscript also have no relevant financial or other relationships to disclose.
References
- Taiwan Cancer Registry. http://tcr.cph.ntu.edu.tw/main.php?Page =N2. Accessed 2016
- Health Promotion Administration, Ministry of Health and Welfare. http://www.hpa.gov.tw/English/ClassPrint.aspx?No =201401280006. Accessed 2016
- Xiong W, Zeng ZY, Xia JH et al A susceptibility locus at chromosome 3p21 linked to familial nasopharyngeal carcinoma. Cancer Res 2004;64:1972-4
- Chie WC, Chang SH, Huang CS et al. () Prognostic factors for the survival of Taiwanese breast cancer patients. J Formos Med Assoc 2002;101:98-103
- Cheng SH, Tsou MH, Liu MC, et al. Unique features of breast cancer in Taiwan. Breast Cancer Res Treat 2000;63:213-23
- Lee JH, Yim SH, Won YJ, et al. Population-based breast cancer statistics in Korea during 1993-2002: incidence, mortality, and survival. J Korean Med Sci 2007;22(Suppl):S11
- Minami Y, Tsubono Y, Nishino Y, et al. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer 2004;108:901-6
- The Research Group for Population-based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1994: estimates based on data from seven population-based cancer registries. Jpn J Clin Oncol 1999;29:361-4
- O'Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol 2015;33:2600-8
- Seferina SC, Lobbezoo DJ, de Boer M, et al. Real-life use and effectiveness of adjuvant trastuzumab in early breast cancer patients: a study of the Southeast Netherlands Breast Cancer Consortium. Oncologist 2015;20:856-63
- Negri E, Zambelli A, Franchi M, et al. Effectiveness of trastuzumab in first-line HER2+ metastatic breast cancer after failure in adjuvant setting: a controlled cohort study. Oncologist 2014;19:1209-15
- van Rooijen JM, de Munck L, Teeuwen GM, et al. Use of trastuzumab for HER2-positive metastatic breast cancer in daily practice: a population-based study focusing on the elderly. Anticancer Drugs 2016;27:127-32
- Leung HW, Chan AL. Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: a meta-analysis of real-world data. Expert Opin Drug Saf 2015;14:1661-71
- Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med 2016;11:123-40
- Murray CJ, Evans DB, Acharya A, et al. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ 2000;9:235-51
- Statistics times: International Monetary Fund World Economic Outlook (April-2015). http://statisticstimes.com/economy/projected-world-gdp-capita-ranking.php. Accessed October 24, 2015
- Dedes KJ, Szucs TD, Imesch P, et al. Cost-effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a model-based analysis of the HERA and FinHer trial. Ann Oncol 2007;18:1493-9
- Essers BA, Seferina SC, Tjan-Heijnen VC, et al. Transferability of model-based economic evaluations: the case of trastuzumab for the adjuvant treatment of HER2-positive early breast cancer in the Netherlands. Value Health 2010;13:375-80
- Hall PS, Hulme C, McCabe C, et al. Updated cost-effectiveness analysis of trastuzumab for early breast cancer: a UK perspective considering duration of benefit, long-term toxicity and pattern of recurrence. Pharmacoeconomics 2011;29:415-32
- Hedden L, O'Reilly S, Lohrisch C, et al. Assessing the real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu positive breast cancer. Oncologist 2012;17:164-71
- Purmonen TT, Pänkäläinen E, Turunen JH, et al. Short-course adjuvant trastuzumab therapy in early stage breast cancer in Finland: cost-effectiveness and value of information analysis based on the 5-year follow-up results of the FinHer Trial. Acta Oncol 2011;50:344-52
- Lidgren M, Wilking N, Jönsson B, et al. Health related quality of life in different states of breast cancer. Quality Life Res 2007;16:1073-81
- Skedgel C, Rayson D, Younis T. The cost-utility of sequential adjuvant trastuzumab in women with Her2/Neu-positive breast cancer: an analysis based on updated results from the HERA Trial. Value Health 2009;12:641-8
- Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-Positive early breast cancer. Value Health 2009;12(3 Suppl):S82-S4
- Shiroiwa T, Fukuda T, Schimozuma K, et al. The model-based cost-effectiveness analysis of 1-year adjuvant trastuzumab treatment: based on 2-year follow-up HERA trial data. Breast Cancer Res Treat 2008;109:559-66
- Garrison LP Jr, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer 2007;110:489-98
- Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica 2013;33:411-17
- Beauchemin C, Letarte N, Mathurin K, et al. A global economic model to assess the cost-effectiveness of new treatments for advanced breast cancer in Canada. J Med Econ 2016;5:1-24