Abstract
Objectives: To estimate clinical outcomes and cost-effectiveness of ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin (OMB/PTV/r + DSV ± RBV) compared with treatment regimens including pegylated interferon (PegIFN) for patients with chronic genotype 1 hepatitis C virus (HCV) infection.
Methods: An Excel spreadsheet Markov model tracking progression through stages of liver disease was developed. Costs and patient utilities for liver disease stages were taken from published studies. Rates of disease progression were based on studies of untreated HCV infection and long-term follow-up of those achieving sustained virologic response (SVR) after drug treatment. Impact of OMB/PTV/r + DSV ± RBV and other drug regimens on progression was estimated through SVR rates from clinical trials. Analyses were performed for treatment-naive and treatment-experienced patients. Impact of alternative scenarios and input parameter uncertainty on the results were tested.
Results: For genotype 1 treatment-naive HCV patients, for OMB/PTV/r + DSV ± RBV, PegIFN + ribavirin (PegIFN/RBV), sofosbuvir + PegIFN/RBV, telaprevir + PegIFN/RBV, boceprevir + PegIFN/RBV, lifetime risk of decompensated liver disease was 5.6%, 18.9%, 7.4%, 11.7%, and 14.9%; hepatocellular carcinoma was 5.4%, 9.2%, 5.7%, 7.0%, and 7.4%; and death from liver disease was 8.7%, 22.2%, 10.4%, 14.8%, and 17.6%, respectively. Estimates of the cost-effectiveness of OMB/PTV/r + DSV ± RBV for treatment-naive and treatment-experienced patients indicated that it dominated all other regimens except PegIFN/RBV. Compared with PegIFN/RBV, the incremental cost-effectiveness ratios were £13,864 and £10,258 per quality-adjusted life-year (QALY) for treatment-naive and treatment-experienced patients, respectively. The results were similar for alternative scenarios and uncertainty analyses.
Limitations: A mixed-treatment comparison for SVR rates for the different treatment regimens was not feasible, because many regimens did not have comparator arms; instead SVR rates were based on those from recent trials.
Conclusions: OMB/PTV/r + DSV ± RBV is a cost-effective oral treatment regimen for chronic genotype 1 HCV infection compared with standard treatment regimens and is estimated to reduce the lifetime risks of advanced liver disease.
Introduction
Hepatitis C is a serious infectious disease caused by the blood-borne hepatitis C virus (HCV). In the UK, estimates of the prevalence of HCV antibodies indicating infection are 0.67% (0.50–0.94%) in those aged 15–59 years, or 203,000 (153,000–286,000) individualsCitation1. About 74% of those with antibodies will continue to have chronic infectionCitation2. There are six different genotypes of hepatitis C. The most common genotypes in the UK are genotype 1 and genotype 3, estimated as 45% and 43.8% of all HCV cases, respectivelyCitation3. Genotypes 2, 4, 5, and 6 make up the rest of the remaining identified genotypes.
The epidemiology of HCV has changed over the last decades, with infusion-related cases no longer a cause of new infections, while intravenous drug use has increasingly become the primary cause of new infectionsCitation4. In their Bayesian evidence synthesis of the epidemiology of HCV in the UK, Harris et al.Citation1 estimated that, of the 150,000 (113,000–226,000) individuals living with chronic HCV infection in the UK, 44% are attributable to current intravenous drug users, 43% to previous intravenous drug users, 7% to white/other ethnicity never injectors, and 5.6% to South Asian never injectors.
Chronic HCV infection is associated with significant morbidity, mortality, and economic burden. If left untreated or if unsuccessfully treated, it can cause cirrhosis and liver cancer in a significant proportion of patients after two to four decades with the chronic infectionCitation5. Once cirrhosis has developed, hepatic decompensation and other potentially fatal complications can occur, requiring frequent hospitalizationsCitation6–14, and liver transplant may be performed as the treatment of choice when end-stage liver disease is reachedCitation15. Chronic HCV infection was also shown to be associated with work impairment, impairment in non-work activities, and reduced quality-of-life in addition to more frequent physician visits than matched controls in the 2010 European National Health and Wellness Survey (n = 57,805)Citation16.
The goal of hepatitis C treatment is viral eradication, measured by a sustained virologic response (SVR). An SVR is defined, according to the latest European Association for the Study of the Liver (EASL) guidelines, as undetectable HCV RNA in the blood either 12 or 24 weeks after a course of therapy is completed as detected by a sensitive molecular method with a lower limit of detection of 615 IU/mLCitation15. Viral eradication prevents the progression of hepatitis C-related liver fibrosis and associated complications such as hepatocellular carcinoma in the majority of cases and delivers “virologic cure” for people with HCV infection. Studies have shown that patients who achieve an SVR are significantly less likely to be hospitalized or die from a liver-related cause than patients who do not achieve an SVRCitation17–21.
Until recently, recommended treatment options for patients in England and Wales all involved the co-administration of pegylated interferon + ribavirin (PegIFN/RBV). Patients with HCV genotype 1 might receive PegIFN/RBV alone or PegIFN/RBV in combination with one of the protease inhibitors (telaprevir [TPV] or boceprevir [BOC]) that act directly on the HCV. While these treatment options provide a cure for some patients, they are not successful in a substantial proportion of those with genotype 1 HCV infectionCitation22–24. These therapies are also associated with a range of limitations, including long and complicated treatment regimens, side-effects that may be hard to tolerate and require extensive monitoring, and the development of treatment-resistant viral mutations, all of which can be associated with treatment failure. The more recent introduction of treatment regimens including a second generation of direct-acting antiviral drugs, such as simeprevir (SIM) or sofosbuvir (SOF) in combination with PegIFN/RBV, has increased the cure rate for those with genotype 1 HCV infection, but still requires the co-administration of PegIFN/RBVCitation25,Citation26. Limitations with all these treatment regimens, especially the inconvenience and side-effects associated with PegIFN therapy that requires weekly injections, are a disincentive for many patients to undergo treatment.
All-oral, interferon-free direct-acting antiviral regimens for chronic HCV genotype 1Citation27 have now been approved for use in the UK, have SVR rates that are over 90%, and are recommended as standard of care in the latest EASL/American Association for the Study of Liver Diseases (AASLD) guidelinesCitation28,Citation29. One of these regimens includes three direct-acting antiviral agents, ombitasvir/paritaprevir/ritonavir, given in combination with dasabuvir with or without ribavirin (OMB/PTV/r + DSV ± RBV). Ritonavir is included as a pharmacokinetic enhancer for the protease inhibitor paritaprevir; ribavirin is included for those with genotype 1a sub-type as well as for those with genotype 1b sub-type with cirrhosis as a non-specific antiviral agent with proven efficacy for HCV. In clinical trials, this regimen has been shown to induce an SVR in most treatment-naive and treatment-experienced patients when treated with a 12-week oral regimen and a 24-week oral regimen in those with genotype 1a cirrhosisCitation30–34. In this economic evaluation for the UK health system, we compare the clinical outcomes, costs, and cost-effectiveness of treatment with OMB/PTV/r + DSV ± RBV with treatment with PegIFN/RBV alone or in combination with another direct-acting antiviral agent in patients with genotype 1 HCV.
Methods
Model structure and assumptions
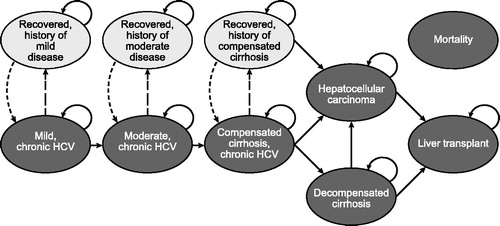
The HCV natural history Markov model used for this analysis takes a National Health Service and Personal Social Service perspective and is based on previously published Markov models with a 1-year cycle time and a 70-year time horizonCitation10,Citation35,Citation36. The model was programmed to be run for 70 years so that lifetime estimates could be generated for entering age cohorts as young as 30 years. The model schematic () shows (1) six health states indicating progressive liver disease (i.e. mild chronic HCV [Metavir stage F0–F1], moderate chronic HCV [Metavir stage F2–F3], compensated cirrhosis [Metavir stage F4], decompensated cirrhosis, hepatocellular carcinoma, and liver transplant); (2) three states representing recovered states, namely SVR (i.e. recovered, history of mild disease; recovered, history of moderate disease; recovered, history of compensated cirrhosis); and (3) death (absorbing health state for liver death and non-liver death). Hepatocellular carcinoma and liver transplant have health states for initial and subsequent years, not represented in the figure. Liver-related death rates are dependent on disease stage, while non-liver-related deaths depend on sex and increase with age. Additional details of the model structure and key assumptions are provided in Supplementary Appendix Table A1.
Patients initiate treatment in the first model year. Patients who do not achieve SVR are at risk of progressive liver disease and assumed to face the same risks of fibrosis progression as untreated patients. With successful treatment, patients achieve SVR; we assume this to be a permanent condition unless re-infectedCitation17–21. This means that patients with an SVR are assumed not to progress to more severe liver disease, other than those with history of compensated cirrhosis, who still face excess risk of hepatocellular carcinoma and liver transplant.
Model inputs
Patient characteristics
presents patient characteristics assumed in the model, including the distribution of patients across stages of liver disease. We assume an average age at model entry of 40 years for a treatment-naive patient and 45 years for a treatment-experienced patientCitation39. The baseline distribution of patients across stages of liver disease, 46.0% mild (F0–F1), 44.0% moderate (F2–F3), and compensated cirrhosis 10.0% (F4), is based on Hartwell et al.Citation10, which in turn uses the data reported from a clinical audit of patients attending a liver clinic at a London teaching hospital for treatment of HCVCitation45. In the base case, we further assume that patients with genotype 1 HCV infection have a body weight ranging between 66–80 kg, 70% are male, and 68.8% have genotype 1a HCVCitation10,Citation37.
Table 1. Summary of non-treatment-specific variables for those with chronic genotype 1 hepatitis C virus.
Disease progression rates for chronic HCV infection
Natural history disease progression probabilities are presented in . We use transition probabilities described in Shepherd et al.Citation35, which were in turn derived from the economic evaluation undertaken alongside the UK Mild Hepatitis C Trial and observational database studyCitation39. The transition probability for hepatocellular carcinoma for those with a history of compensated cirrhosis and an SVR is taken from a long-term follow-up study of patients achieving an SVR after treatment with Peg/IFN/RBVCitation41.
Comparators included in the model
Comparators included in the model were treatment regimens currently used in the UK and for which sufficient clinical trial data were available to allow patient segmentation required by the model. For the treatment-naive population, we compared OMB/PTV/r + DSV ± RBV against PegIFN/RBV, SOF + PegIFN/RBV, TPV + PegIFN/RBV, and BOC + PegIFN/RBV in interferon-eligible patients. For the treatment-experienced population, we compared OMB/PTV/r + DSV ± RBV against PegIFN/RBV and TPV + PegIFN/RBV, since only these regimens had trial data available for all three patient sub-groups for whom therapy with PegIFN/RBV had failed: (1) previously not responded, (2) partially responded, and (3) responded and relapsed.
Treatment-specific clinical outcomes
Treatment-specific clinical outcomes including SVR rates, adverse event rates, and duration of therapy for the base-case analyses for different treatment regimens are presented in (SVR rates), (adverse event rates), and (mean duration of therapy) for genotype 1 treatment-naive and treatment-experienced patients stratified by fibrosis level. These data were obtained directly from clinical trials for the treatment regimens (a listing of trials used to derive the clinical outcomes data for each drug regimen is provided in Supplementary Appendix Table A2). Where multiple trials for OMB/PTV/r + DSV ± RBV providing estimates of SVR on the same population were available, the data were pooled. Data on SVR for treatment-experienced patients were calculated as the weighted average of those who had previously not responded, partially responded, or responded and relapsed using the percentages of each patient type presented in . Adverse event rates were included for five clinically significant treatment-related adverse events: anemia, rash, depression, grade 3 or 4 neutropenia, and grade 3 or 4 thrombocytopenia (). Trial discontinuation rates based on pre-specified stopping rules and the indicated treatment duration were combined to estimate the mean duration on treatment for each treatment regimen ().
Table 2. Sustained virologic response rates for those with chronic genotype 1 hepatitis C virus infection by treatment regimen and fibrosis stage: base-case values.
Table 3. Adverse event rates for those with chronic genotype 1 hepatitis C virus infection by treatment regimen and fibrosis stage: base-case values.
Table 4. Treatment duration for those with chronic genotype 1 hepatitis C virus infection.
Treatment-related costs
All model costs are presented in 2013 UK pounds. The costs for each drug regimen () were obtained from the Monthly Index of Medical SpecialtiesCitation50 for all marketed drug regimens. A weight-based dose of 1000 mg per day was assumed for ribavirin based on an assumed weight range between 66–80 kg. Total treatment cost is a function of the regimen’s weekly cost and the mean treatment duration presented in .
Table 5. Treatment-related costs for those with chronic genotype 1 hepatitis C virus infection.
In addition to drug acquisition costs, costs for monitoring patients while they are receiving drug treatment and drug-related adverse events were included in the model (). The monitoring costs were derived for PegIFN treatmentCitation35 and adapted for novel direct-acting antiviral regimens based on expert opinion to reflect shortened treatment duration of PegIFN-containing therapy (SOF + PegIFN/RBV) or treatment with a PegIFN-free regimen (OMB/PTV/r + DSV ± RBV). Because the direct-acting antiviral drugs are relatively new, we assumed that monitoring requirements for an interferon-free direct-acting antiviral regimen would be the same as those for an interferon-based regimen of equal duration. Costs for each drug-related adverse event were estimated using resource use estimates and unit costs based on expert opinion or used in previous studiesCitation52–54.
Disease-related costs
The costs associated with chronic HCV infection in each health state were obtained from two sources ()Citation10,Citation44. All costs are updated to current values using the Hospital and Community Health Services IndexCitation51. The same costs are applied to treatment-naive and treatment-experienced patients. We assumed that all recovered patients require life-long monitoring after achieving an SVR, irrespective of their initial fibrosis stage.
Disease-state health utility
Disease-state health utility () reflects the expected annual health-related quality-of-life associated with chronic HCV over the complete disease course. Our base-case disease-state utility values are taken from Hartwell et al.Citation10. Experience of SVR, captured in the model by the “recovered” states, has been shown to positively improve baseline health-related quality-of-life. Based on Wright et al.Citation39, SVR from mild fibrosis, moderate fibrosis, or compensated cirrhosis is associated with a 0.05 increase in health utility.
Treatment-related health utility
Treatment-related health utility (Supplementary Appendix Table A3) reflects the change in health-related quality-of-life over the duration of each treatment regimen that is due to (1) utility decrease due to treatment-related adverse effects and inconvenience and (2) utility increase due to improvement in disease symptoms. Treatment-related health utility was derived from the EQ-5D-5L questionnaires included in the SAPPHIRE, PEARL, and TURQOUISE trials for OMB/PTV/r + DSV ± RBV and from the published literature for the comparator regimensCitation55 and is applied during the first year of the model time horizon only.
Analyses
We first ran the model over the full time horizon to generate estimates of lifetime probabilities of clinical outcomes of liver disease and discounted (at 3.5%) lifetime liver-related healthcare costs and quality-adjusted life-years (QALYs) using the base-case input parameter estimates described above for both treatment-naive and treatment-experienced patients. We then conducted three types of sensitivity analyses: (1) scenario analyses to account for initiating treatment in different compensated fibrosis stages (mild, moderate, and cirrhosis or no antiviral treatment); (2) deterministic sensitivity analyses; and (3) probabilistic sensitivity analysis.
Supplementary Appendix Tables A4 and A5 present the input parameter value ranges tested in the deterministic sensitivity analysis and the statistical moments and probability distributions tested in the probabilistic sensitivity analysis. The results of the one-way sensitivity analysis were presented in tornado diagrams. Probabilistic sensitivity analysis was undertaken for treatment-naive and treatment-experienced patients for all treatment regimens. Given that multiple treatment options were available in segments, we generated cost-effectiveness acceptability curves, monetizing QALYs to employ a net monetary benefits framework. For each cost-effectiveness acceptability curve, 500 simulations were drawn from the variables’ assumed distributions. Treatment history, genotype, background death rate, and regimen prices were not varied in the one-way and probabilistic sensitivity analyses.
Validation
The model, which was similar in structure to previously published Markov modelsCitation10,Citation35,Citation36, was validated internally as well as externally. The model was validated for internal accuracy and consistency through a thorough check of all formulae and macros by independent researchers not involved in the model creation. External validation included cross-validation of the results with recently published models as well as comparison of the model results for untreated patients with those from observational data.
Results
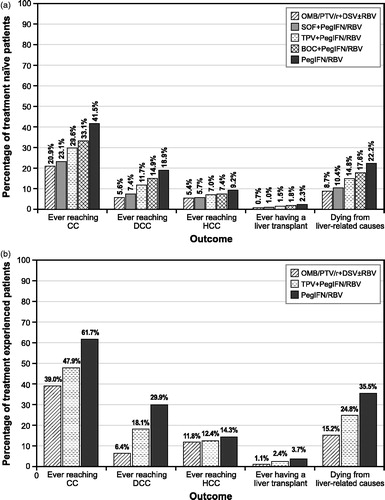
present the clinical outcomes for the different treatment regimens using the base-case input parameter values. In both treatment-naive and treatment-experienced patients, the percentage ever reaching the more advanced liver disease or dying from a liver-related cause is lowest with OMB/PTV/r + DSV ± RBV compared with the other treatment regimens.
Figure 2. Base-case clinical outcomes for chronic genotype 1 hepatitis C virus infected, (a) treatment-naive patients and (b) treatment-experienced patients. BOC, boceprevir; CC, compensated cirrhosis; DCC, decompensated cirrhosis; DSV, dasabuvir; HCC, hepatocellular carcinoma; IFN, interferon; OMB, ombitasvir; PegIFN, pegylated interferon; PTV, paritaprevir; r, ritonavir; RBV, ribavirin; SOF, sofosbuvir; TPV, telaprevir.
presents the corresponding discounted lifetime costs, QALYs, cost-effectiveness ratios compared with PegIFN/RBV, and incremental cost-effectiveness ratios for all treatment regimens. In treatment-naive and treatment-experienced patients, the discounted lifetime costs, life-years, and QALYs are lowest for the PegIFN/RBV regimen. The cost-effectiveness ratios for all comparators compared with PegIFN/RBV are all below £20,000–£30,000/QALY, threshold values commonly used by NICE in the UK. The incremental cost-effectiveness ratios show that OMB/PTV/r + DSV ± RBV dominates when compared with SOF + PegIFN/RBV or shows extended dominanceFootnote† when compared with all other regimens except PegIFN/RBV and has an incremental cost/QALY ratio of £13,864 when compared with PegIFN/RBV for the treatment-naive population and £10,258 when compared with PegIFN/RBV for the treatment-experienced population.
Table 6. Base-case economic results for chronic genotype 1 hepatitis C virus infected, treatment-naïve, and treatment-experienced patients.
Supplementary Appendix Tables A6 and A7 present the clinical and economic results, respectively, of the scenario analyses, where treatment of patients with either mild fibrosis, moderate fibrosis, or compensated cirrhosis was explored. For both treatment-naive and treatment-experienced patients with no antiviral treatment, those with mild fibrosis have a lower probability of ever experiencing advanced liver disease than those with moderate or compensated cirrhosis. For all three patient sub-groups, treatment with OMB/PTV/r + DSV ± RBV provides the greatest reduction in the probability of advanced liver disease compared with other treatment regimens, with the exception of treatment-experienced patients with compensated cirrhosis (Supplementary Appendix Table A6). For treatment-naive patients with mild or moderate fibrosis, OMB/PTV/r + DSV ± RBV dominates, extendedly dominates, or is cost-effective compared with all other treatment regimens and compared with no antiviral treatment (Supplementary Appendix Table A7). For compensated cirrhosis, OMB/PTV/r + DSV ± RBV dominates, extendedly dominates, or is cost-effective compared with most regimens and compared with no antiviral treatment but not compared with SOF + PegIFN/RBV in treatment-naive compensated cirrhosis patients, where the cost-effectiveness ratio is £36,198/QALY (Supplementary Appendix Table A7). For treatment-experienced patients with mild or moderate fibrosis or compensated cirrhosis, OMB/PTV/r + DSV ± RBV extendedly dominates or is cost-effective compared with all treatment regimens and compared with no treatment at a threshold of £20,000/QALY (Supplementary Appendix Table A7).
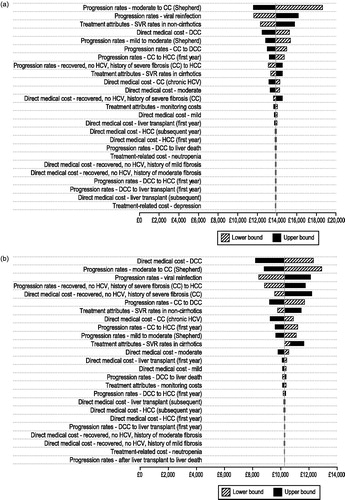
present the results of the deterministic sensitivity analysis for the cost-effectiveness analysis of OMB/PTV/r + DSV ± RBV compared with PegIFN/RBV (the one non-dominated treatment regimen) varying the base-case parameter values across their assumed ranges. The results are most sensitive to disease progression rates (lower rate of progression, higher incremental cost-utility ratio), direct medical care costs (lower costs, higher incremental cost-utility ratio), and difference in SVR rates (higher difference in SVR, lower incremental cost-utility ratio) in the treatment-naive and treatment-experienced population. However, in all cases the cost-effectiveness of OMB/PTV/r + DSV ± RBV compared with PegIFN/RBV remains below £20, 000/QALY.
Figure 3. Tornado diagram: deterministic sensitivity analysis for (a) treatment-naive and (b) treatment-experienced patients with chronic genotype 1 hepatitis C infection: OMB/PTV/r + DSV ± RBV compared with pegIFN/RBV. CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; OMB/PTV/r + DSV ± RBV, ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin; PegIFN/RBV, pegylated interferon + ribavirin; SVR, sustained virologic response.
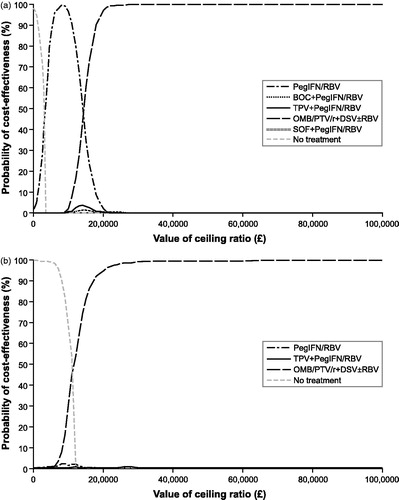
present the cost-effectiveness acceptability curve results of the probabilistic sensitivity analysis for the treatment-naive and treatment-experienced populations. All comparator treatments examined were included in each cost-effectiveness acceptability curve as well as no treatment. For treatment-naive patients, OMB/PTV/r + DSV ± RBV is the optimal treatment regimen if the payer is willing to pay more than £15,000/QALY. If they are willing to pay less than £15,000/QALY, then either PegIFN/RBV or no treatment is the optimal treatment strategy. For treatment-experienced patients, OMB/PTV/r + DSV ± RBV is the optimal treatment regimen if the payer is willing to pay more than £13,000/QALY. If they are willing to pay less than £13,000/QALY, then no treatment is the optimal treatment strategy. SOF + PegIFN/RBV, TPV + PegIFN/RBV, or BOC + PegIFN/RBV are not optimal treatment regimens at any payer willingness-to-pay values.
Figure 4. Cost-effectiveness acceptability curves for (a) treatment-naive and (b) treatment-experienced patients with chronic genotype 1 hepatitis C infection. BOC, boceprevir; OMB/PTV/r + DSV ± RBV, ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin; PegIFN/RBV, pegylated interferon + ribavirin; SOF, sofosbuvir; TPV, telaprevir.
Discussion
Compared with all comparators, OMB/PTV/r + DSV ± RBV demonstrated superior efficacy, less treatment-related disutility, and better tolerability, resulting in an estimated lower probability of progressing to advanced stages of liver disease or dying from liver-related causes and higher QALY gains. The results of the base-case cost-effectiveness analysis showed that OMB/PTV/r + DSV ± RBV dominated SOF + PegIFN/RBV, extendedly dominated TPV + PegIFN/RBV and BOC + PegIFN/RBV, and was cost-effective compared with PegIFN/RBV, with cost-effectiveness ratios well below generally accepted threshold values for both treatment-naive and treatment-experienced patients. The probabilistic sensitivity analysis estimated that OMB/PTV/r + DSV ± RBV was the optimal regimen for willingness-to-pay thresholds as low as £15,000/QALY for both treatment-naive and treatment-experienced patients.
The results of the scenario analyses indicated that, although the results varied among those with mild or moderate fibrosis and with compensated cirrhosis, OMB/PTV/r + DSV ± RBV remained dominant, extendedly dominant, or cost-effective compared with all interferon-based comparator regimens with treatment initiation at mild and moderate fibrosis levels for both treatment-naive and treatment-experienced patients. This result was also observed for treatment initiation in compensated cirrhosis except for the comparison in treatment-naive patients with SOF + PegIFN/RBV where the ICER for OMB/PTV/r + DSV ± RBV was £36 198. Although the proportion of patients ever experiencing decompensated liver disease, hepatocellular carcinoma, liver transplant, or liver-related death was higher when initiating treatment in those with compensated cirrhosis compared with initiating treatment in those with mild fibrosis, treatment with OMB/PTV/r + DSV ± RBV was predicted to reduce the probability of patients dying from liver-related causes across all fibrosis stages.
The internal validation process confirmed the accuracy of the model formulae and macros. External validation of the model with natural history studies found that the rate of disease progression for those not treated was slower in our model (11.7% with cirrhosis after 20 years) than that estimated in meta-analyses of observational data studies (20.0% after 20 yearsCitation56 and 21.9% after 20 yearsCitation57), but consistent (21.2% after 30 years) with the rate of progression used in previous models (20% after 30 years)Citation10,Citation35,Citation39. Cross-validation with other published models including OMB/PTV/r + DSV ± RBV found generally consistent results. A US study using the same model structure and clinical input valuesCitation58 showed similar results for OMB/PTV/r + DSV ± RBV compared with PegIFN/RBV and other direct-acting antiviral agent + PegIFN/RBV regimens for those with genotype 1 HCV infection. In addition, this study included other oral direct-acting antiviral agent regimens and showed that the sofosbuvir-containing oral regimens had similar long-term outcomes to OMB/PTV/r + DSV ± RBV, but OMB/PTV/r + DSV ± RBV had lower costs and higher QALYs and so dominated these other regimens. The cost-effectiveness of oral regimens for different levels of fibrosis is shown in the Zhang et al.Citation59 modeling study of the cost-effectiveness of sofosbuvir-based treatments in the US. Their study showed that OMB/PTV/r + DSV ± RBV dominates all sofosbuvir-based regimens for those without cirrhosis and is cost-effective compared with SOF + PegIFN/RBV, but more expensive and slightly less effective than SOF + ledipasvir for those with cirrhosis.
Although all the new treatment regimens with direct-acting antiviral drugs have been shown to be cost-effective relative to PegIFN/RBVCitation60–64, their high costs and the large number of people potentially indicated for treatment have raised questions about the appropriate patients to receive treatment. The National Health Service recently issued a report commissioning treatment with the second-generation direct-acting antiviral regimens for chronic HCV infection only for those with either compensated or decompensated cirrhosisCitation65. Nevertheless, the results of our model showed that treating patients with mild or moderate fibrosis or treatment-experienced patients with compensated cirrhosis with OMB/PTV/r + DSV ± RBV is cost-effective using generally accepted threshold values. In addition, those with compensated cirrhosis who achieve an SVR with treatment are still at increased risk of hepatocellular carcinoma compared with the general population, indicating the clinical value of earlier treatment.
The model presented in this article has several strengths. It has been developed in line with previously published models, using similar input parameters to model disease progression and health state utilities; this ensured consistency with previous assessments and facilitates the ease of comparisons and validations of the model results. In addition, the model contains three recovered health states representing virologic cure of chronic HCV, which are differentiated by the patients’ stage of liver disease prior to treatment; this stratification facilitates modeling of the subsequent risk of progressive liver disease important for recovered patients with history of compensated cirrhosis. Lastly, we were able to use data on SVR rates by fibrosis stage from the clinical trials of OMB/PTV/r + DSV ± RBV and other regimens, where available, allowing the model to estimate the clinical and economic outcomes by disease stage.
The model also has several limitations. There is very limited information on the baseline characteristics of patients with chronic HCV in the UK; in particular, we found no information on the baseline characteristics for patients in the sub-groups used in the model; we used baseline characteristics from the overall treatment-naive and treatment-experienced genotype 1 patients enrolled in clinical trials. Clinical experts regarded these assumptions as reasonable and in line with their clinical experience; similar assumptions have been made in NICE assessments in chronic HCV, most recently in the appraisals of sofosbuvir and simeprevir.
There are numerous trials of PegIFN/RBV documenting SVRs in genotype 1 patients, published over a number of years. Originally, our intention was to model efficacy inputs using estimates from a mixed-treatment comparison for all treatment regimens. However, as a mixed-treatment comparison was not feasible in this disease area because of lack of comparator arms in the SOF + PegIFN/RBV and OMB/PTV/r + DSV ± RBV clinical trials, PegIFN/RBV SVR estimates for treatment-naive and treatment-experienced patients in our model base case are based on the SVRs of patients receiving PegIFN/RBV in the more recent ADVANCE and REALIZE trials comparing TPV + PegIFN/RBV with PegIFN/RBV aloneCitation47,Citation49,Citation66.
Finally, recent EMA label modifications allow OMB/PTV/r + DSV to be given without ribavirin in those with HCV genotype 1b. However, more recent data from TURQUOISE III indicate higher SVR rates for OMB/PTV/r + DSV without ribavirin in patients with genotype 1b cirrhosis than those used in the current analyses when given with ribavirin. Combining this information with a lower regimen cost and a better safety profile (e.g. lower anemia rate) would result in a more favorable cost-effectiveness profile for OMB/PTV/r + DSV.
In summary, our model found that OMB/PTV/r + DSV ± RBV prevents more cases of advanced liver disease than the other treatment regimens tested. It is also a cost-effective treatment for all genotype 1 treatment-naive and treatment-experienced patients as well as for sub-groups initiating treatment with mild fibrosis, moderate fibrosis, or compensated cirrhosis.
Transparency
Declaration of funding
Funding was provided by AbbVie Global to Medicus Economics to develop the cost-effectiveness model.
Declaration of financial/other interests
SJ and SV are employees of Medicus Economics Inc., a consulting company that conducts economic evaluations in a variety of therapeutic areas for pharmaceutical companies. HP is a contractor to Medicus Economics. IF is an employee of AbbVie UK, and JS and DM are employees of AbbVie Global, the manufacturers of ombitasvir/paritaprevir/ritonavir and dasabuvir. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary file
Download MS Word (98.5 KB)Acknowledgments
The authors acknowledge the technical writing assistance of Josephine Mauskopf, an employee of RTI Health Solutions, in the preparation of this manuscript. Funding was provided by AbbVie to RTI Health Solutions to provide this technical writing assistance.
Notes
*Assume that there are two drug regimens, A and B. Drug regimen B is said to dominate drug regimen A if drug regimen B has a lower cost and is more effective (higher QALYs) than drug regimen A.
†Assume that there are three drug regimens A, B, and C, with drug regimen C more effective (higher QALYs) and more costly than drug regimen B and drug regimen B more effective (higher QALYs) and more costly than drug regimen A. Then drug regimen C is said to extendedly dominate drug regimen B if the cost-effectiveness ratio for drug regimen C compared with drug regimen A is more favorable (lower ratio) than the cost-effectiveness ratio for drug regimen B compared with drug regimen A.
References
- Harris RJ, Ramsay M, Hope VD, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health 2012;22:187-92
- Bruggmann P, Berg T, Ovrehus ALH, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepatitis 2014;21(Suppl 1):5-33
- Brant LJ, Ramsay ME, Tweed E, et al.; Sentinel Surveillance of Hepatitis Testing Group. Planning for the healthcare burden of hepatitis C infection: hepatitis C genotypes identified in England, 2002-2007. J Clin Virol 2010;48:115-9
- Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008;48:148-62
- Seeff LB. The history of the “natural history” of hepatitis C (1968-2009). Liver Int 2009;29(1 Suppl):89-99
- Stahmeyer JT, Rosol S, Bert F, et al. Cost of treating hepatitis C in Germany: a retrospective multicenter analysis. Eur J Gastroenterol Hepatol 2014;26:1278-85
- Xu T, Tong X, Leidner AJ. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health Aff (Millwood) 2014;33:1728-35
- Tandon N, Balart LA, Laliberte F, et al. Impact of completing chronic hepatitis C (CHC) treatment on post-therapy healthcare cost. J Med Econ 2014;17:862-71
- Manos MM, Darbinian J, Rubin J, et al. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm 2013;19:438-47
- Hartwell D, Jones J, Baxter L, et al. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 2011;15:i-xii, 1–210
- McDonald SA, Hutchinson SJ, Bird SM, et al. The growing contribution of hepatitis C virus infection to liver-related mortality in Scotland. Euro Surveill 2010;15:19562
- Neal KR, Trent Hepatitis C Study Group, Ramsay S, et al. Excess mortality rates in a cohort of patients infected with the hepatitis C virus: a prospective study. Gut 2007;56:1098-104
- McDonald SA, Hutchinson SJ, Bird SM, et al. A record linkage study of the development of hepatocellular carcinoma in persons with hepatitis C infection in Scotland. Br J Cancer 2008;99:805-10
- McDonald SA, Hutchinson SJ, Bird SM, et al. Hospitalization of hepatitis C-diagnosed individuals in Scotland for decompensated cirrhosis: a population-based record-linkage study. Eur J Gastroenterol Hepatol 2010;22:49-57
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392-420
- Vietri J, Prajapati G, El Khoury AC. The burden of hepatitis C in Europe from the patients’ perspective: a survey in 5 countries. BMC Gastroenterol 2013;13:16
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011;52:889-900
- Veldt BJ, Saracco G, Boyer N, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut 2004;53:1504-08
- Morisco F, Granata R, Stroffolini T, et al. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol 2013;19:2793-8
- Papastergiou V, Stampori M, Lisgos P, et al. Durability of a sustained virological response, late clinical sequelae, and long-term changes in aspartate aminotransferase to the platelet ratio index after successful treatment with peginterferon/ribavirin for chronic hepatitis C: a prospective study. Eur J Gastroenterol Hepatol 2013;25:798-805
- Koh C, Heller T, Haynes-Williams V, et al. Long-term outcome of chronic hepatitis C after sustained virological response to interferon-based therapy. Aliment Pharmacol Ther 2013;37:887-94
- Shepherd J, Brodin H, Cave C, et al. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2004;8:iii-iv, 1–125
- Yang D, Liang HJ, Li D, et al. The efficacy and safety of telaprevir-based regimens for treating chronic hepatitis C virus genotype 1 infection: a meta-analysis of randomized trials. Intern Med 2013;52:653-60
- Ferenci P, Reddy KR. Impact of HCV protease-inhibitor-based triple therapy for chronic HCV genotype 1 infection. Antivir Ther 2011;16:1187-201
- Druyts E, Lorenzi M, Toor K, et al. Network meta-analysis of direct-acting antivirals in combination with peginterferon-ribavirin for previously untreated patients with hepatitis C genotype 1 infection. QJM 2015;108:299-306
- Welch NM, Jensen DM. Pegylated interferon based therapy with second-wave direct-acting antivirals in genotype 1 chronic hepatitis C. Liver Int 2015;35(1 Suppl):11-17
- Sharma SA, Feld JJ. Management of HCV in cirrhosis- a rapidly evolving landscape. Curr Gastroenterol Rep 2015;17:443
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015;63:199-236
- American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America (ISDA). Recommendations for testing, managing, and treating hepatitis C [Internet]. 2016. http://www.hcvguidelines.org. Accessed April 19, 2016
- Feld J, Kowdley K, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594-603
- Ferenci P, Bernstein D, Lalezari J, et al.; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014;370:1983-92
- Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973-82
- Zeuzem S, Jacobson I, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1604-14
- Andreone P, Colombo M, Enejosa J, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014;147:359-65
- Shepherd J, Jones J, Hartwell D, et al. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007;11:1-205
- Grishchenko M, Grieve RD, Sweeting MJ, et al.; Trent HCV Study Group. Cost-effectiveness of pegylated interferon and ribavirin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care 2009;25:171-80
- Harris KA, Gilham C, Mortimer PP, et al. The most prevalent hepatitis C virus genotypes in England and Wales are 3a and 1a. J Med Virol 1999;58:127-31
- National Institute for Health and Care Excellence (NICE). Simeprevir for the treatment of genotype 1 and genotype 4 chronic hepatitis C [Single Technology Appraisal ID668], 18 September 2014. https://www.nice.org.uk/guidance/TA331/documents/hepatitis-c-chronic-simeprevir-id668-evaluation-report2. Last accessed May 18, 2016
- Wright M, Grieve R, Roberts J, et al.; UK Mild Hepatitis C Trial Investigators. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10:1-113
- Grieve R, Roberts J, Wright M, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006;55:1332-8
- Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol 2010;52:652-7
- Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463-72
- Bennett WG, Inoue Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997;127:855-65
- Backx M, Lewszuk A, White JR, et al. The cost of treatment failure: resource use and costs incurred by hepatitis C virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat 2014;21:208-15
- Foster GR, Goldin RD, Main J, et al. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ 1997;315:453-8
- Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-87
- Jacobson IM, McHutchison JG, Dusheiko G, et al.; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011;364:2405-16
- Poordad F, McCone J Jr, C Bacon BR, et al.; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195-206
- Zeuzem S, Andreone P, Pol S, et al.; REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med 2011;364:2417-28
- Monthly Index of Medical Specialties (MIMS) [Internet]. Available at http://www.mims.co.uk/. Last accessed May 24, 2014
- Curtis L. Unit costs of health and social care. Personal Social Services Research Unit, 2013
- Thorlund K, Druyts E, El Khoury AC, et al. Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection. Clinicoecon Outcomes Res 2012;4:349-59
- National Institute for Health and Care Excellence (NICE). Sofosbuvir for chronic hepatitis C, 18 March, 2014. https://www.nice.org.uk/guidance/TA330/documents/hepatitis-c-chronic-sofosbuvir-evaluation-report4. Last accessed May 18, 2016
- National Institute for Health and Care Excellence (NICE). Depression: the treatment and management of depression in adults (updated edition). Clinical Practice Guideline 90. British Psychological Society, Leicester, UK and the Royal College of Psychiatrists, London, UK, 2010
- Younossi ZM, Stepanova M, Henry L, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol 2014;60:741-7
- Thein HH, Yo Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-38
- Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809-16
- Saab S, Parisé H, Virabhak S, et al. Cost-effectiveness of currently recommended direct-acting antiviral treatments in patients infected with genotypes 1 or 4 hepatitis C virus in the United States. J Med Econ 2016:1-42
- Zhang S, Bastian ND, Griffin PM. Cost-effectiveness of sofosbuvir-based treatments for chronic hepatitis C in the US. BMC Gastroenterology 2015;15:98
- Saab S, Gordon SC, Park M, et al. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther 2014;40:657-75
- Petta S, Cabibbo G, Enea M, et al.; WEF Study Group. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology 2014;59:1692-705
- San Miguel R, Gimeno-Ballester, Mar J. Cost-effectiveness of protease inhibitor based regimens for chronic hepatitis C: a systematic review of published literature. Expert Rev Pharmacoecon Outcomes Res 2014;14:387-402
- Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 2014;46(5 Suppl):S186-S96
- Pfeil AM, Reich O, Guerra IM, et al. Cost-effectiveness analysis of sofosbuvir compared to current standard treatment in Swiss patients with chronic hepatitis C. PLoS One 2015;10:e0126984
- National Health Service (NHS) England Clinical Reference Group for Infectious Diseases. Clinical commissioning policy statement: treatment of chronic hepatitis C in patients with cirrhosis [Internet]. National Health Services, 2015. Presented at HepDART 2011, Kauai, HI, USA, December 4–8. http://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/06/hep-c-cirrhosis-polcy-statmnt-0615.pdf. Accessed October 6, 2015
- Kauffman RS, Muir AJ, Nelson DR, et al. Review activity/safety: telaprevir in combination with peginterferon alfa-2a and ribavirin increased sustained virologic response in genotype 1 chronic HCV. Conference reports for the National AIDS Treatment Advocacy Project. 2011. Presented at HepDART 2011, Kauai, HI, USA, December 4–8. http://www.natap.org/2011/hepDART/hepDART_03.htm. Accessed October 6, 2015