Abstract
Objective: To assess the cost-effectiveness of exenatide 2 mg once-weekly (EQW) compared to dulaglutide 1.5 mg QW, liraglutide 1.2 mg and 1.8 mg once-daily (QD), and lixisenatide 20 μg QD for the treatment of adult patients with type 2 diabetes mellitus (T2DM) not adequately controlled on metformin.
Methods: The Cardiff Diabetes Model was applied to evaluate cost-effectiveness, with treatment effects sourced from a network meta-analysis. Quality-adjusted life years (QALYs) were calculated with health-state utilities applied to T2DM-related complications, weight changes, hypoglycemia, and nausea. Costs (GBP £) included drug treatment, T2DM-related complications, severe hypoglycemia, nausea, and treatment discontinuation due to adverse events. A 40-year time horizon was used.
Results: In all base-case comparisons, EQW was associated with a QALY gain per patient; 0.046 vs dulaglutide 1.5 mg; 0.102 vs liraglutide 1.2 mg; 0.043 vs liraglutide 1.8 mg; and 0.074 vs lixisenatide 20 μg. Cost per patient was lower for EQW than for liraglutide 1.8 mg (−£2,085); therefore, EQW dominated liraglutide 1.8 mg. The cost difference per patient between EQW and dulaglutide 1.5 mg, EQW and liraglutide 1.2 mg, and EQW and lixisenatide 20 μg was £27, £103, and £738, respectively. Cost per QALY gained with EQW vs dulaglutide 1.5 mg, EQW vs liraglutide 1.2 mg, and EQW vs lixisenatide 20 μg was £596, £1,004, and £10,002, respectively. In the probabilistic sensitivity analysis, the probability that EQW is cost-effective ranged from 76–99%.
Conclusion: Results suggest that exenatide 2 mg once-weekly is cost-effective over a lifetime horizon compared to dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD, and lixisenatide 20 μg QD for the treatment of T2DM in adults not adequately controlled on metformin alone.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder that manifests when the body is unable to effectively utilize insulin to regulate blood glucose levelsCitation1. Elevated blood glucose levels (i.e. hyperglycemia) result in diabetes-related complications including cardiovascular disease, renal disease, limb amputation, retinopathy, and deathCitation2. T2DM has become a global health burden. The International Diabetes Federation estimated that globally 387 million people aged 20–79 years (8.5%) were living with diabetes in 2014, with a projection of 592 million (9.9%) for 2035Citation3. Healthcare expenditure due to diabetes accounted for 11% of the total global healthcare spend in 2014. Approximately €111 billion was spent on healthcare due to diabetes in Europe in 2014, accounting for almost one-third of global healthcare expenditureCitation3. In the UK, it is currently estimated that the National Health Service (NHS) spends ∼£10 billion on diabetes, which represents 10% of its total annual budgetCitation4.
In recent years, new therapeutic agents have expanded the treatment options for T2DM beyond metformin, sulfonylurea, and insulin. Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are subcutaneously administered agents that stimulate insulin secretionCitation5. The GLP-1 RA exenatide is currently marketed in two formulations: a solution for twice-daily injection providing either 5 or 10 μg of exenatide (exenatide BID); and a prolonged-release once-weekly injection that provides 2 mg of exenatide (exenatide QW). The long-acting formulation contains the active ingredient of the original exenatide BID formulation dispersed in microspheres of medical-grade poly-(d,l-lactide-co-glycolide) (PLG) in an aqueous formulationCitation6. The efficacy of exenatide QW was demonstrated in the DURATION clinical trial program, where it was compared to exenatide BID, sitagliptin, pioglitazone, insulin glargine, metformin, and liraglutideCitation7. However, there is a lack of head-to-head clinical trials comparing exenatide QW to newer GLP-1 RAs such as dulaglutide and lixisenatide. In the absence of direct comparisons within a clinical trial setting, an estimate of the efficacy and safety of exenatide QW, relative to other GLP-1 RAs, can be determined from a network meta-analysis. A recent network meta-analysis was conducted to estimate the relative efficacy and tolerability of exenatide QW compared to other GLP-1 RAs for patients with T2DM not adequately controlled on metforminCitation8.
Exenatide QW is indicated for treatment of T2DM in combination with metformin, sulfonylurea (SU), thiazolidinedione (TZD), metformin and SU, or metformin and TZD in adults who have not achieved adequate glycemic control on maximally tolerated doses of these oral therapiesCitation9. The National Institute for Health and Care Excellence (NICE) new guidelines for the management of T2DM in adultsCitation10 place the GLP-1 RAs in the second intensification of drug treatment if triple therapy with metformin and two other oral drugs were not effective, not tolerated, or contraindicated. Specifically, GLP-1 RAs should be considered in combination with metformin and SU for adults with T2DM who are not adequately controlled with triple oral therapyCitation10. In addition, hypoglycemia and weight gain are common adverse effects from treatment with insulin and SUs that create a barrier to optimizing treatment and complicate the management of T2DMCitation11,Citation12. Efforts by patients to lose weight as part of a therapeutic lifestyle program are undermined by therapies that lead to weight gain, such as SUs, TZDs, and insulinCitation11. The treatment-associated weight gain may exacerbate diabetes disease progression, augments insulin resistance, and negatively impacts cardiovascular risk factors including hypertension and dyslipidemia, since over 85% of patients with T2DM are overweight or obeseCitation13.
Economic evaluation studies comparing novel health technologies and interventions are an integral component of health technology assessments. Cost-effectiveness analyses are critical for healthcare planning and decision-making. Regulatory and reimbursement requirements of many countries consider evidence of economic value along with clinical efficacy in support of their decision-making procedures for the pricing and/or reimbursement of health technologiesCitation14,Citation15. Today, decision-makers include governments, third-party insurers, healthcare delivery systems, healthcare professionals, and patients. The present study aimed to assess the cost-effectiveness of exenatide QW vs dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD, and lixisenatide 20 μg QD for the treatment of T2DM in adults inadequately controlled on metformin alone and in whom other oral drugs are not effective, sub-optimal, or contraindicated. The study utilized treatment effects from an NMACitation8 and was conducted from the perspective of the UK NHS.
Methods
Cost-effectiveness model
The previously published and validated Cardiff Diabetes Model, a fixed-time-increment stochastic simulation model, was applied as the basis for the current economic analysisCitation16. Risk equations, based on the UK Prospective Diabetes Study (UKPDS) 68 were used to estimate long-term micro- and macrovascular complications including congestive heart failure, ischemic heart disease, myocardial infarction, stroke, amputation, blindness, and end stage renal disease (ESRD), as well as diabetes-related mortality and other mortalityCitation17. The occurrence of diabetes-related complications was determined by the time- and treatment-dependent evolution of modifiable risk factors, including HbA1c, body mass index, the ratio of total and high density lipoprotein cholesterol, and systolic blood pressure. All-cause mortality was based on gender-specific life tables for the UK. Patients were simulated over a total period of 40 years, indicative of a lifetime horizon for T2DM. At the end of each cycle, the incidence of fatal and non-fatal complications was determined. Once a fatal event occurred, life years and quality-adjusted life years (QALYs) were updated and the simulation ended. The model included drug acquisition costs, costs related to treatment administration, macro- and micro-vascular complications, hypoglycemia, and nausea as an adverse event (AE). The model assumed that quality-of-life was affected by macro- and micro-vascular complications, hypoglycemic episodes, nausea, and body mass index (BMI). The health outcomes were measured in terms of QALY gained, life years gained, and events avoided.
In the base case analysis, 1000 cohorts of 1000 individual patients were modeled to ensure stability in the simulation results. The primary outcome measure used in this cost-effectiveness analysis was the incremental cost-effectiveness ratio (ICER), measured as cost per QALY gained with exenatide QW vs the comparator. A discount rate of 3.5% was applied to both costs and health effects, as recommended by the National Institute for Health and Care Excellence (NICE) in the UKCitation18.
Treatment sequences
The initial treatments in the model were exenatide QW, dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD, and lixisenatide 20 μg QD as add-on to metformin (2500 mg per day). Treatment-specific reductions in HbA1c were applied in the first year of the simulation. This decline in HbA1c was followed by a natural progression of HbA1c, which was modeled via the implementation of the UKPDS 68 equationCitation17. Patients escalated to Neutral Protamine Hagedorn (NPH) insulin (57.2 units per day) added on top of metformin (2,252 mg per day) at the HbA1c switching threshold of 7.5%, following the health economic evaluation approach which underpins the new NICE guideline for management of T2DM in adultsCitation19. Besides loss of glycemic control, patients could also switch to the next line of treatment due to treatment discontinuation. Once treated with NPH insulin, patients stayed on this treatment for the remaining time-horizon of the model or until death. Applying a similar next line of treatment in all modeled treatment strategies ensures that the incremental model results are solely attributable to the relative differences in efficacy and tolerability associated with the initial treatment.
Patient population and treatment effects
Baseline characteristics () for age, duration of diabetes, systolic blood pressure, and weight were sourced from a recent NMA conducted to estimate the relative efficacy and tolerability of exenatide QW compared to other GLP-1 receptor agonists (RAs) for the treatment of adult patients with T2DM not adequately controlled on metforminCitation8. This NMA included a total of 6677 patients from 14 randomized controlled trials of 24 weeks (±6 weeks) treatment duration. Exenatide QW obtained a statistically significant reduction in HbA1c relative to lixisenatide 20 μg QD. No other comparisons of exenatide QW to other GLP-1 RAs were statistically significant for change in HbA1c. There was no statistically significant difference in weight change between exenatide QW and the other GLP-1 RAs. Exenatide QW was associated with a lower risk of nausea compared to all GLP-1 RAs, except exenatide 5 μg BID (none of these differences were statistically significant). Risk of discontinuation due to adverse events was lower for exenatide QW than for dulaglutide 1.5 mg QW, and liraglutide 1.2 mg and 1.8 mg QD, and higher for EQW than for lixisenatide 20 μg QD and exenatide 5 μg and 10 μg BID (none of these differences were statistically significant). In the cost-effectiveness model, the baseline HbA1c value of patients entering the model was assumed to be 7.5%, which follows the health economic evaluation conducted by NICECitation19. Other baseline characteristics were sourced from the UKPDS 33 studyCitation20.
Table 1. Baseline patient characteristics applied in the model.
Treatment-specific reductions in HbA1c were sourced from the NMA and are shown in . Treatment-specific probabilities of nausea and treatment discontinuation due to AE were also sourced from the NMA and applied during the first model cycle (). The level of body mass index (BMI) has been shown to impact health-related quality-of-lifeCitation21. In the model, treatment-specific effects on body weight were applied in the first year of the simulation (). For instance, exenatide QW was associated with a weight change of −2.04 kg. It was assumed that this effect was maintained for 4 years, which was substantiated by the 6-year results from the extension of the DURATION-1 studyCitation22 and the final 3-year follow-up of the DURATION-3 studyCitation23. After the period of weight maintenance, it was assumed that the initial weight loss would be fully regained in a linear manner within 1 year, with weight returning to its baseline value at the time of switch to treatment with NPH insulin. For other GLP-1 RAs a similar assumption was made; an initial weight loss in the first year of the simulation (), a period of weight maintenance while on treatment, and initial weight loss fully regained within 1 year, with weight returning to its baseline value at the time of switch to NPH insulin. The weight gain associated with NPH insulin () was assumed not be lost in subsequent years.
Table 2. Treatment efficacy and tolerability applied in the model.
Costs
Economic data used in the model included costs for drug treatment, diabetes-related complications, severe hypoglycemia, nausea, and treatment discontinuation due to AE (). The UKPDS 84 studyCitation24 was utilized as the key source for costs associated with diabetes-related complications. These costs were indexed to 2014 prices using the Hospital and Community Health Services Pay and Price indexCitation25. Drug acquisition costs for the initial treatments were sourced from the Monthly Index of Medical Specialties database of prescription and generic drugs (www.mims.co.uk; last accessed in January 2016), whilst recommended treatment doses, the use of consumables (e.g. needles), and the cost associated with NPH insulin mainly followed those reported in the NICE health economic evaluationCitation19.
Table 3. Cost inputs applied in the model.
Utility values
Utility decrements used in the model are shown in . The UKPDS 62 studyCitation26 was utilized as the source for EQ-5D utility decrements for diabetes-related complications. The utility decrement for ESRD was sourced from a study conducted with the Health Outcomes Data Repository (HODaR) by Currie et al.Citation27. The impact of body weight on utility values was derived from a study where a linear utility change of one unit in BMI was reportedCitation21. The quality-of-life impact associated with patients’ fear of hypoglycemia following symptomatic and severe episodes of hypoglycemia was incorporated in the model as wellCitation28.
Table 4. Utility decrements applied in the model.
Sensitivity analyses
Extensive deterministic univariate and probabilistic multivariate sensitivity analyses were carried out to assess the impact of uncertainty of model inputs on estimated results. Within the univariate deterministic sensitivity analyses the efficacy and tolerability parameters of exenatide QW and comparators (i.e. HbA1c reduction, weight loss, treatment discontinuation due to AE, risk of nausea) and BMI-related utility changes were varied within their 95% confidence intervals. Utility decrements for diabetes-related complications were altered by ±10%, and non-drug costs were varied by ±25%. In order to estimate second order uncertainty, a probabilistic sensitivity analysis (PSA) was performed, where 1,000 cohorts of 30,000 patients were simulated. Where standard errors were not reported, parameter variability was assumed to be a percentage of the mean value in line with the magnitude of other standard errors for similar variables.
In addition, a number of scenario analyses were conducted to explore the uncertainty of model inputs and assumptions that could not be fully captured within the deterministic and probabilistic sensitivity analyses. One of the main model assumptions was that the HbA1c reductions of the NMACitation8, where the mean baseline HbA1c value of the included studies was 8.2%, could be applied in the base case analysis where the baseline HbA1c was set at 7.5%. The potential impact of this assumption was addressed in two alternative scenarios. In one, both the baseline HbA1c value and the threshold for treatment escalation were set at 8.2%. In a second scenario, the treatment effects observed in the NMA were down-adjusted (by a factor of 7.5%/8.2%) to reflect the lower baseline HbA1c value in the model. A scenario was conducted where the BMI-related utility changes were derived from a study by Lane et al.Citation29, where the utility change of one unit increase and decrease in BMI was −0.0472 and 0.0171, respectively. The impact of including treatment effects on systolic blood pressure was also analysed, as was the inclusion of BMI-related healthcare costs. In the model base case, there were no costs associated with changes in BMI, but higher body mass may be an indication of more complications and, thus, increased healthcare resources and costs. BMI-related cost estimates were derived from a published study that analyzed the relationship between BMI and prescription costs in a UK healthcare setting (the Counterweight Program)Citation30.
Results
Base case
Exenatide QW was associated with an incremental cost of £27 compared to dulaglutide 1.5 mg QW ( and ), which was caused by the higher total treatment cost of the exenatide QW arm. Exenatide QW was associated with a QALY gain compared to dulaglutide 1.5 mg QW (incremental benefits of 0.046 QALYs), driven by a better tolerability profile of exenatide QW (). The incremental cost per QALY gained with exenatide QW compared to dulaglutide 1.5 mg QW was predicted at £596, well below the cost-effectiveness threshold range used by NICE (£20,000–30,000 per QALY gained) ().
Table 5. Results (discounted) of exenatide QW and comparators: Base case and probabilistic sensitivity analysis.
Table 6. Disaggregated base case incremental cost (£) calculated for exenatide QW vs comparators.
Compared to liraglutide 1.2 mg QD, exenatide QW was associated with an incremental cost of £103 ( and ). This was caused by the duration of initial treatment with exenatide QW or liraglutide 1.2 mg QD before patients switched to NPH insulin, which was associated with a lower drug acquisition cost (). More precisely, duration of initial treatment was longer with exenatide QW, which was the result of better glycemic control combined with improved tolerability compared to liraglutide 1.2 mg QD; also reflected by the estimated incremental benefits of 0.102 QALYs (). The incremental cost per QALY gained with exenatide QW compared to liraglutide 1.2 mg QD was predicted at £1,004, well below the cost-effectiveness threshold range used by NICE (£20,000–30,000 per QALY gained) ().
Exenatide QW was associated with a lower lifetime cost compared to liraglutide 1.8 mg QD (incremental cost of −£2,085) ( and ), mainly because drug acquisition cost of exenatide QW is lower than that of liraglutide 1.8 mg QD (). Exenatide QW was associated with a QALY gain (incremental benefits of 0.043 QALYs) (), which was driven by the slightly better glycemic control combined with improved tolerability compared to liraglutide 1.8 mg QD. Thus, in this comparison exenatide QW was the dominant treatment strategy, i.e. lower costs and QALYs gained compared to liraglutide 1.8 mg QD.
Finally, in the comparison to lixisenatide 20 μg QD the incremental cost was £738 ( and ), which was caused by the higher drug acquisition cost of exenatide QW (). Exenatide QW was associated with an incremental benefit of 0.074 QALYs (), which was attributable to the larger treatment effect on HbA1c and weight compared to lixisenatide 20 μg QD. The cost per QALY gained with exenatide QW compared to lixisenatide 20 μg QD was £10,002 (), well below the cost-effectiveness threshold range of £20,000–30,000 per QALY gained.
Sensitivity analyses
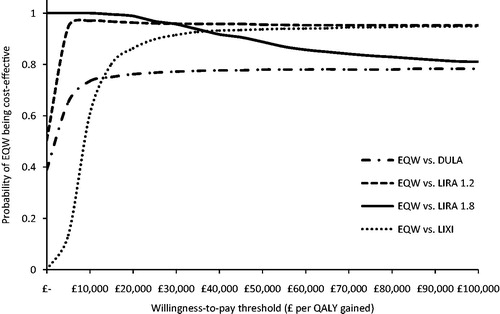
The deterministic univariate sensitivity analyses revealed that point estimates for incremental costs were most sensitive to variations in the treatment-specific HbA1c reduction and risk of treatment discontinuation due to AE (full results from sensitivity analyses are available from the authors upon request). QALYs were also most sensitive to changes in drug tolerability inputs. For instance, changing the probability of discontinuing treatment with liraglutide 1.8 mg QD from its baseline value of 0.13 to its lower 95% CI limit of 0.0076 resulted in a decrease in incremental QALYs from 0.043 to −0.022 and in incremental costs from −£2,085 to −£2,466. Results from the PSA are presented in . In the PSA, the probability that exenatide QW is cost-effective ranged from 76–99% across all comparisons, assuming a willingness-to-pay threshold of £20,000 per QALY gained (). In all scenario analyses conducted, exenatide QW remained dominant or cost-effective, with the calculated ICER remaining below the cost-effectiveness threshold range of £20,000–30,000 per QALY gained. Overall, it was concluded from the univariate sensitivity and scenario analyses that the base case estimates were robust to changes in the main model parameters and assumptions.
Discussion
This study investigated the cost-effectiveness of exenatide QW against other GLP-1 RAs, including dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD, and lixisenatide 20 μg QD in adults inadequately controlled on metformin alone and in whom other oral drugs are not effective, not tolerated, or contraindicated. The results suggested that exenatide QW is a cost-effective option in all individual treatment comparisons. The main model drivers included better efficacy in terms of HbA1c and body weight reduction, and/or a better tolerability associated with exenatide QW. The conducted sensitivity and scenario analyses suggested that the results were robust to changes in the model parameters and assumptions.
Several cost-effectiveness studies examining exenatide QW are published in the literature. However, to the best of our knowledge, the current study is the first simulation of the cost-effectiveness of exenatide QW compared to newer GLP-1 RAs. A number of studies modeled insulin glargine or exenatide BID as the comparator treatmentCitation31–33. A cost-consequence study compared exenatide QW with sitagliptin or pioglitazoneCitation34. Wang et al.Citation35 compared the short-term (6-month and 1-year) cost-effectiveness of exenatide QW against liraglutide 1.8 mg QD. In this US-based study the cost-effectiveness was evaluated and assessed as the cost per 1% reduction in HbA1c. The relative clinical efficacy and tolerability was derived from the DURATION-6 trialCitation36 and a NMACitation37. The liraglutide 1.8 mg QD strategy had an incremental cost per 1% reduction in HbA1c of $4773 and $27,179, using clinical data from DURATION-6 (which directly compared exenatide QW and liraglutide 1.8 mg QD) and the NMA, respectively. These short-term cost-effectiveness results could not be compared with our long-term simulations of costs and QALYs because of differences in outcome measure and time horizon. However, as part of this study, an indicative long-term simulation (unpublished) was conducted utilizing DURATION-6 data. In that simulation, liraglutide 1.8 mg QWD was associated with higher costs and a QALY gain compared to exenatide QW. The resulting cost per QALY gained with liraglutide 1.8 mg QD vs exenatide QW was well above the cost-effectiveness threshold range of £20,000–30,000 per QALY gained. It could, therefore, be reasoned that liraglutide 1.8 mg QD may not be cost-effective compared to exenatide QW, based on the results of the DURATION-6 trial.
The use of the Cardiff Diabetes Model adds to the strength of the current analyses. The Cardiff Diabetes Model has been validated, published, and used in several multi-country studies involving patients from Australia, European Union, Canada, and the USCitation38–41. In addition, most of the data inputs, such as costs and utilities associated with micro- and macro-vascular complications, have been widely used and accepted by NICE (including recent single technology appraisals for exenatide QWCitation42, liraglutideCitation43, as well as Clinical Guideline 87Citation44). In addition, extensive PSA, univariate, and scenario sensitivity analyses were conducted to investigate the impact of uncertainty associated with model parameters and assumptions on model outcomes. Therefore, it is believed that the model results provide a valid and robust estimation of the cost-effectiveness of exenatide QW compared to other GLP-1 RAs, with uncertainty around the results appropriately addressed.
One of the key model assumptions is required because of the lack of head-to-head data comparing exenatide QW with dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD,Footnotea and lixisenatide 20 μg QD. The relative efficacy and tolerability of exenatide QW and comparators were, therefore, based on a NMACitation8. A NMA can provide useful evidence regarding the difference in treatment effects among competing interventions which otherwise would be lackingCitation45. The underlying major assumption is that all trials involved in the evidence network of the NMA are comparable in terms of study design and study population, and, thus, the results of each trial can be compared. To minimize the heterogeneity of the population included in the NMA, given the different efficacies of various anti-hyperglycemic agents and to ensure similarity regarding the population’s clinical spectrum of T2DM, only trials that examined patients who had failed metformin monotherapy, and who received a GLP-1 RA as add-on therapy to metformin only were included. The reader is referred to Kayaniyil et al.Citation8 for further information on the NMA. Furthermore, it was assumed that the treatment efficacy and tolerability estimated from the NMA, where the mean baseline HbA1c value observed in the included studies was 8.2%, could be applied in the base case scenario where baseline HbA1c was set at 7.5%. This may be viewed as a strong postulation. In acknowledgement of this, the impact of this assumption was extensively tested in scenario analyses. The results of these scenario analyses did not alter the conclusions of the base case scenario. Furthermore, in a separate sensitivity analysis of the NMA itself, whereby baseline HbA1c was adjusted for, no significant covariate effect for baseline HbA1c was identifiedCitation8, suggesting that the assumptions made in the current cost-effectiveness analyses may be appropriate. The present cost-effectiveness analysis did not evaluate albiglutide, even though it was included in the NMA because albiglutide has not been approved in the UK.
Assumptions were also required regarding the extrapolation of treatment effects on body weight. For exenatide QW, a weight lowering effect was applied in the first year of treatment and was assumed to be maintained for an additional 4 years. This assumption was supported by clinical evidence from the extension of the DURATION-1 trial, which showed that weight loss associated with exenatide QW was sustained to 6 years in patients who continued treatmentCitation22. Furthermore, in the final follow-up of the DURATION-3 trial, the weight loss associated with exenatide QW was sustained for 3 yearsCitation23. Similar assumptions regarding the maintenance of weight effect and regain of weight were conservatively made for the comparators included in the cost-effectiveness analysis, although the available evidence on weight loss maintenance relating to the comparator treatments is not as robust. Finally, the annual cost of NPH insulin was based on a fixed dose, whereas insulin is dosed per kilogram body weight in routine clinical practice. Utilizing results from the NMA, the current analyses assumed that body weight differs depending on the initial treatment administered to patients when they enter the model. However, the fixed insulin dose assumption had limited impact on the model results, since body weight was assumed to return to its baseline value at the time of switch to treatment with NPH insulin.
Conclusion
The results of this study suggest that exenatide QW is cost-effective compared to dulaglutide 1.5 mg QW, liraglutide 1.2 mg QD, liraglutide 1.8 mg QD, and lixisenatide 20 μg QD for the treatment of T2DM in adults inadequately controlled on metformin alone and in whom other oral drugs are not effective, sub-optimal, or contraindicated. The results were robust to changes in the main model parameters and assumptions. To the best of our knowledge, this study is the first simulation of the cost-effectiveness of exenatide QW compared to newer GLP-1 RAs. As such, this study provides new information that can help decision-makers identify the GLP-1 RA that offers the greatest economic value.
Transparency
Declaration of funding
This research was supported by AstraZeneca Pharmaceuticals.
Declaration of financial/other relationships
LHC and BGV are employees of Pharmerit International and received research funds from AstraZeneca to conduct the analysis. MC was an employee of Pharmerit International at the time of this research and is now employee of UCB Biopharma SPRL. DG, SG, and BK are employees and stockholders of AstraZeneca. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Kathleen M. Fox, PhD of Strategic Healthcare Solutions, LLC provided medical writing support and received funds from AstraZeneca.
Notes
a The DURATION-6 trialCitation36 compared the efficacy and safety of exenatide QW with liraglutide 1.8 mg QD in patients with T2DM. DURATION-6 was not included in the NMA, since patients were on a variety of oral anti-diabetic agents prior to and throughout the trial and, therefore, it did not meet the inclusion criteria of the NMA that was performed for GLP-1 RAs as add-on to metformin alone.
References
- Ahmed KA, Muniandy S, Ismail IS. Type 2 diabetes and vascular complications: a pathophysiologic view. Biomed Res 2010;21:147-55
- Hayes AJ, Leal J, Gray AM, et al. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013;56:1925-33
- International Diabetes Federation. IDF Diabetes Atlas sixth edition. 2014. http://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. Accessed November 12, 2015
- Hex N, Bartlett C, Wright D, et al. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabetes Med 2012;29:855-62
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Diabetes Care 2015;38:140-9
- DeYoung MB, MacConell L, Sarin V, et al. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide). Diabetes Technol Ther 2011;13:1145-54
- Grimm M, Han J, Weaver C, et al. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the DURATION trials. Postgrad Med 2013;125:47-57
- Kayaniyil S, Lozano-Ortega G, Bennett HA, et al. A network meta-analysis comparing exenatide once weekly with other GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus. Diabetes Therapy 2016;7:27-43
- EMA. Bydureon. Summary of product characteristics. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. Accessed December 10, 2015
- National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2015. http://www.nice.org.uk/guidance/ng28. Accessed December 10, 2015
- Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. J Am Med Assoc 2010;303:1410-18
- Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes. A population-based study of health service resource use. Diabetes Care 2003;26:1176-80
- Daousi C, Casson IF, Gill GV, et al. Prevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factors. Postgrad Med J 2006;82:280-4
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health 2009;12:409-18
- Ramsey SD, Wilke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II – An ISPOR good research practices task force report. Value Health 2015;18:161-72
- McEwan P, Evans M, Bergenheim K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes Obes Metab 2010;12:623-30
- Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013. http://www.nice.org.uk/article/pmg9. Accessed November 13, 2015
- National Institute of Health and Care Excellence. Appendix F: Full Health Economics Report. type 2 diabetes in adults: management. draft for consultation. 2015. http://www.nice.org.uk/guidance/gid-cgwave0612/documents/type-2-diabetes-appendix-f2. Accessed November 13, 2015
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53
- Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ 2005;14:217-30
- Henry RR, Klein EJ, Malloy J, et al. DURATION-1 extension: efficacy and tolerability of exenatide once weekly over 6 years in patients with T2DM. Diabetes 2014;63(suppl 1):A247
- Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2014;2:464-73
- Alva M, Gray A, Mihaylova B, et al. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabetes Med 2015;32:459-66
- PSSRU. Unit costs of health & social care. 2014. http://www.pssru.ac.uk/project-pages/unit-costs/2014. Accessed November 13, 2015
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Mak 2002;22:340-9
- Currie CJ, McEwan P, Peters JR, et al. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Lane S, Levy A, Mukherjee J, et al. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin 2014;30:1267-73
- Counterweight Project Team. Influence of body mass index on prescribing costs and potential cost savings of a weight management programme in primary care. J Health Serv Res Policy 2008;13:158-66
- Samyshkin Y, Guillermin AL, Best JH, et al. Long-term cost-utility analysis of exenatide once weekly versus insulin glargine for the treatment of type 2 diabetes patients in the US. J Med Econ 2012;15(Suppl 2):6-13
- Fonseca T, Clegg J, Caputo G, et al. The cost-effectiveness of exenatide once weekly compared with exenatide twice daily and insulin glargine for the treatment of patients with type two diabetes and body mass index ≥30 kg/m(2) in Spain. J Med Econ 2013;16:926-38
- Beaudet A, Palmer JL, Timlin L, et al. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ 2011;14:357-66
- Guillermin A, Lloyd A, Best J, et al. Long-term cost-consequence analysis of exenatide once weekly vs sitagliptin or pioglitazone for the treatment of type 2 diabetes patients in the United States. J Med Econ 2012;15:654-63
- Wang B, Roth JA, Nguyen H, et al. The short-term cost-effectiveness of once-daily liraglutide versus once-weekly exenatide for the treatment of type 2 diabetes mellitus in the United States. PLoS One 2015;10:e0121915
- Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013;381:117-24
- Scott DA, Boye KS, Timlin L, et al. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab 2013;15:213-23
- The Mount Hood 4 Modeling Group. Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46
- Granström O, Bergenheim K, McEwan P, et al. Cost-effectiveness of saxagliptin (Onglyza®) in type 2 diabetes in Sweden. Prim Care Diabetes 2012;6:127-36
- Scottish Medicine Consortium. Dapagliflozin for the Treatment of Type 2 Diabetes, 2014. http://www.scottishmedicines.org.uk/SMC_Advice/Advice/799_12_dapagliflozin_Forxiga/Briefing_note_dapagliflozin_Forxiga_Resubmission. Accessed November 13, 2015
- Cummins E, Scott N, Rothnie K, et al. Evidence Review Group report; dapagliflozin for the treatment of type 2 diabetes. Aberdeen HTA Group, 2013. http://www.nice.org.uk/guidance/ta288. Accessed November 13, 2015
- National Institue of Health and Care Excellence. Exenatide prolonged-release suspension for injection in combination with oral antidiabetic therapy for the treatment of type 2 diabetes, 2012. http://www.nice.org.uk/guidance/ta248. Accessed November 13, 2015
- National Institute of Health and Care Excellence. Liraglutide for the treatment of type 2 diabetes mellitus, 2010. http://www.nice.org.ul/guidance/ta203. Accessed November 13, 2015
- National Institute for Health and Clinical Excellence. NICE clinical guideline 87. Type 2 diabetes: the management of type 2 diabetes, 2009. http://www.nice.org.uk/guidance/cg87. Accessed November 13, 2015
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14:417-28
- Baboolal K, McEwan P, Sondhi S, et al. The cost of renal dialysis in a UK setting—a multicentre study. Nephrol Dial Transplant 2008;23:1982-9
- Hammer M, Lammert M, Mejías SM, et al. Costs of managing severe hypoglycaemia in three European countries. J Med Econ 2009;12:281-90