Abstract
Objective: Given the substantial economic and health burden of cardiovascular disease and the residual cardiovascular risk that remains despite statin therapy, adjunctive therapies are needed. The purpose of this model was to estimate the cost-effectiveness of high-purity prescription eicosapentaenoic acid (EPA) omega-3 fatty acid intervention in secondary prevention of cardiovascular diseases in statin-treated patient populations extrapolated to the US.
Methods: The deterministic model utilized inputs for cardiovascular events, costs, and utilities from published sources. Expert opinion was used when assumptions were required. The model takes the perspective of a US commercial, third-party payer with costs presented in 2014 US dollars. The model extends to 5 years and applies a 3% discount rate to costs and benefits. Sensitivity analyses were conducted to explore the influence of various input parameters on costs and outcomes.
Results: Using base case parameters, EPA-plus-statin therapy compared with statin monotherapy resulted in cost savings (total 5-year costs $29,393 vs $30,587 per person, respectively) and improved utilities (average 3.627 vs 3.575, respectively). The results were not sensitive to multiple variations in model inputs and consistently identified EPA-plus-statin therapy to be the economically dominant strategy, with both lower costs and better patient utilities over the modeled 5-year period.
Limitations: The model is only an approximation of reality and does not capture all complexities of a real-world scenario without further inputs from ongoing trials. The model may under-estimate the cost-effectiveness of EPA-plus-statin therapy because it allows only a single event per patient.
Conclusion: This novel model suggests that combining EPA with statin therapy for secondary prevention of cardiovascular disease in the US may be a cost-saving and more compelling intervention than statin monotherapy.
Introduction
Cardiovascular disease (CVD) is the cause of one in every three deaths in the US, and results in direct and indirect costs exceeding $300 billion annuallyCitation1. The annual incidence of recurrent myocardial infarction (MI) and stroke are 210,000 and 185,000, respectively, in the USCitation1. Thus, despite best evidence-based treatment strategies, including high-dose statin therapy, there remains a high residual risk of cardiovascular (CV) events and significant health and economic burdensCitation2. Adjunctive therapies shown to reduce CV events when added to statin therapy include the omega-3 fatty acid eicosapentaenoic acid (EPA) and ezetimibeCitation3–6.
The Japan Eicosapentaenoic Acid Lipid Intervention Study (JELIS) was a long-term CV outcomes trial that explored the benefits of EPA in combination with statin therapy compared with statin monotherapy in adult patients with hypercholesterolemiaCitation3. The major finding of an analysis of the secondary prevention population from JELIS was a significant 23% relative risk reduction in the incidence of major coronary events (MCE) in the EPA-plus-statin group (8.7% vs 10.7%, adjusted hazard ratio [HR] = 0.77, 95% confidence interval [CI] = 0.63–0.96, p = 0.017, number needed to treat [NNT] = 49)Citation5. Additionally, among 1050 patients with prior MI, the incidence of MCE in the EPA-plus-statin group was significantly reduced by 27% (adjusted HR = 0.73, 95% CI = 0.54–0.98, p = 0.033, NNT = 19)Citation5. As no significant changes were observed in low-density lipoprotein cholesterol, total cholesterol, and high-density lipoprotein cholesterol levels in the secondary prevention population, the findings from JELIS suggest that treatment strategies, such as EPA, that provide benefits beyond lipid effects may also be important in reducing atherosclerotic CV riskCitation3–5.
It has been suggested that pleiotropic effects may be an important feature for interventional strategies to successfully reduce CV riskCitation7. The broad range of potentially beneficial CV effects of EPA includes those on arrhythmia, inflammation, blood pressure, and lipidsCitation8. Furthermore, EPA is incorporated into membrane phospholipids and atherosclerotic plaques and has beneficial effects in multiple aspects of atherosclerosis including antioxidant properties; endothelial function; effects on macrophages, monocytes, and foam cells; inflammation; plaque progression, formation, and vulnerability; and thrombus formationCitation9. Recent evidence suggests that EPA may also increase high-density lipoprotein anti-inflammatory properties and augment cholesterol efflux capacity from arterial wall macrophagesCitation10.
The drug used in JELIS was EPA ethyl ester (Epadel®, Mochida Pharmaceuticals Co., Ltd., Tokyo, Japan)Citation3,Citation11. In the US, the high-purity EPA drug icosapent ethyl (Vascepa®, Amarin Pharma Inc., Bedminster, NJ) is approved as an adjunct to diet and exercise to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemiaCitation12. Vascepa is not approved by the US Food and Drug Administration to reduce the risk of coronary heart disease. The effect of Vascepa on the risk of CV mortality and morbidity has not been determined.
Icosapent ethyl is the ethyl ester of EPACitation12 and is the same active moiety used in JELIS. Icosapent ethyl is the only prescription omega-3 fatty acid product approved in the US that does not contain docosahexaenoic acid (DHA). While EPA does not tend to raise low-density lipoprotein cholesterol (LDL-C), products containing DHA may complicate treatment strategies in patients with dyslipidemia, as DHA may raise LDL-CCitation13–18.
As JELIS was conducted in an exclusively Japanese population, we developed a model to explore the potential cost-effectiveness of high-purity prescription EPA-plus-statin therapy compared with statin therapy alone. We utilized event rates from the JELIS secondary prevention cohort for the statin + EPA arm to approximate reduced rates likely to be seen when extrapolated to a US population, and we utilized event rates for the statin-only arm, mainly from the Cholesterol Treatment Trialists’ (CTT) CollaborationCitation19 analyses (and others as described in the Methods) as they may more closely represent event rates from the US. Sensitivity analyses were also conducted to determine scenarios in which EPA added to statin was not cost-effective. Of note, there is precedence for extrapolating clinical trial results from a purely Japanese population to a US population. The full panel report of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults considered the results from the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) trial to be generalizable to the US population. MEGA was one of four trials that formed the basis for the ACC/AHA recommendation for statin use in a primary prevention populationCitation20. Additionally, data from two PROBE-designed trials, the Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes trial and the Japanese Primary Prevention Project, were incorporated into a systematic evidence review for the US Preventive Services Task Force regarding aspirin for the primary prevention of cardiovascular eventsCitation21.
Methods
Model design
The cost-effectiveness model was a deterministic decision analytic model designed to estimate total costs associated with the use of pharmacologic therapy and with major adverse CV and cerebrovascular events (MACCE) for statin-treated patients with and without prescription EPA use per quality-adjusted life year or per unit of utility. The model takes the perspective of a US commercial third-party payer and extends to 5 years.
Identification and definition of input parameters
The model includes inputs for CV events, costs, and utilities from multiple published sources. Many of the input parameters were obtained directly from peer-reviewed literature (). Default values were provided as shown in the tables, but the user has the option to vary inputs to other pre-specified values or to add a value not included as a default.
Table 1. Base case input parameters: clinical events.
Table 2. Base case input parameters: costs.
Table 3. Base case input parameters: utilities.
Clinical events
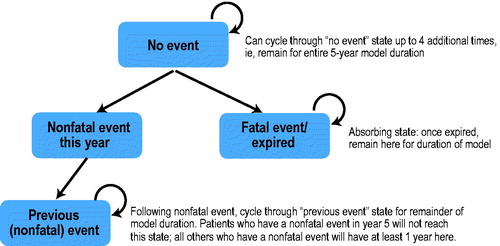
depicts the CV event flow in the model. MACCE included in the model were sudden cardiac death, fatal MI, non-fatal MI, unstable angina, coronary artery bypass grafting (CABG), percutaneous transluminal coronary angioplasty (PTCA), and stroke. With the exception of stroke, the model used JELIS secondary prevention data for the EPA-plus-statin arm and values from CTT higher-intensity vs lower-intensity statin trials for the statin monotherapy arm ()Citation5,Citation19,Citation22–26. The model assumes that the event rates, except for stroke, will be similar to those from the JELIS secondary prevention study in the EPA-plus-statin arm, as the high plasma levels of EPA achieved by patients in the JELIS trial (170 μg/mL)Citation27 have also been achieved by a high-dose purified prescription EPA product in US patientsCitation28,Citation29. Stroke rates for the JELIS secondary prevention data are higher than US rates; therefore, stroke event rates from the secondary prevention studies in the CTT analysisCitation19 were utilized in the model. The percentage reduction in relative risk for stroke between the EPA-plus-statin arm vs the statin monotherapy arm was modeled to be similar to the percentage reduction in relative risk for stroke between the EPA-plus-statin arm vs the statin monotherapy arm observed in the JELIS secondary prevention strata.
also presents other assumptions that were made in order to address two data gaps: in the statin monotherapy group, fatal MI events were assumed to be 1.0% and unstable angina was assumed to be 7.5%. These assumptions were informed by published literatureCitation23,Citation26. Of note, the JELIS data combined CABG and PTCA without presenting data on each end-point separatelyCitation5. Because there are different costs for the two procedures, with CABG costs being substantially higher, attributing costs to these events required making an assumption about the distribution between the two. Using the base case, the model was set at 35% for CABG and 65% for PTCA (informed by the FREEDOMCitation24 and SYNTAXCitation25 studies). However, the user can select from pre-specified percentages, with CABG representing 100%, 65%, 35%, or 0% of interventions. The model also includes a user-defined field wherein the user can enter any number between 0% and 100% as the proportion of all CABG interventions. In this way, although a default value is identifiable in the model, the user can specify any value.
The JELIS secondary prevention report provided the number of events over the 4.6-year mean follow-up of the study, but it did not present data on the distribution of events during each year; therefore, assumptions about the distribution were required to populate the model. Based on a review of published studies among patient groups with risk factorsCitation19,Citation22–25, the per annum distribution of CABG/PTCA interventions were considered separately from other MACCE in the model. The default distribution for CABG/PTCA was assigned as 33% in each of the first 2 years, with the remaining 33% distributed evenly over the final 3 years. For other MACCE, the default distribution was equal over each of the 5 years. For both distributions, there are also built-in options to use alternative values built into the model (e.g. front-loaded with 25% in each of the first 2 years, with the remainder evenly distributed, or back-loaded with 15% in each of the first 2 years, with the remainder evenly distributed) as well as a functionality for users to vary the distribution over the time period as desired.
Costs
Published literature was assessed to identify the costs of treatment for the MACCE that were included as end-points in the JELIS secondary prevention strata: sudden cardiac death, fatal MI, non-fatal MI, unstable angina, CABG/PTCA, and strokeCitation24,Citation25,Citation30–39. These costs and sources are presented in .
In addition to costs for each event, the model applied costs for the year or years following each event. For stroke and non-fatal MI, the ratio of first-year costs vs following-year costs were applied based on published data, while for revascularization and unstable angina, estimates from specific studies were used (). Wholesale acquisition costs were used for prescription drug costs. Costs were inflated to 2014 values using price index information as reported by the Bureau of Labor Statistics (Series ID CUUR0000SAM, medical care) and were discounted at a rate of 3% per annum.
Utilities
Based on the literature, we estimated utilities for each event; the base case utility values are presented in Citation40,Citation41. Utilities were decremented by a specified fraction for the year during which an event occurred. For example, the quality-of-life decrement associated with stroke was 37.1%. The model assumed that events would be evenly distributed throughout the year, so, on average, half the year would be affected; thus, the model applied this decrement for half of the year, while the other half was considered to remain at the baseline value (e.g. 0.78). If the patient died during the year, a utility value of 0 was assigned for half the year. In the years subsequent to an event, the model assumes that patients’ utility values increase halfway back to baseline (i.e. the midpoint value between the utility during the event year and the baseline utility). As in the case of cost inputs, values were discounted at a rate of 3% per annum.
Calculations
The cost-effectiveness model was developed in Microsoft Excel® 2010 and all accompanying calculations were executed therein. For each model input type (i.e. pharmaceutical costs, event costs, utilities), the same process is implemented. Based on user inputs about events and their distribution over time, the outcomes for each year are calculated based on the proportion of patients who fall into each category. For example, the proportion of patients who have a non-fatal MI in year 1 of the model is used to apply a cost associated with MI to those patients and to decrement their utility values accordingly. These patients then remain in the model and accrue a lower cost (compared with the event cost) in each subsequent year as well as a higher utility (compared with the event year) for the remaining years in the model. A patient who suffers a fatal event during year 1 is assigned a cost for the event year as well as a lower utility value for that year. After the initial year, that patient is then removed from the analysis and receives no additional costs or utilities. The process is repeated for each subsequent year, with the pool of patients decreasing each year as fatal events occur. Patients who have additional non-fatal events are assigned adjusted costs and utilities according to their previous event (i.e. increased costs and decreased utilities; see and ), but they are assigned the same cost for pharmaceutical agents annually as long as they remain alive. Incremental cost-effectiveness is calculated as the difference in total cost over 5 years (for EPA-plus-statin therapy minus statin monotherapy) divided by the difference in effectiveness over 5 years (expressed as total utilities for EPA-plus-statin therapy minus total utilities for statin monotherapy).
Sensitivity analyses
For each input parameter, sensitivity analyses were conducted assuming a range of plausible and extreme values to examine the influence of each parameter on the model and to test the robustness of the model. Rates, timing of events, patient utilities, costs of events, and overall assumptions were varied and tested. In addition to modifying individual model input parameters, two alternative scenarios were developed that involved different values for all clinical inputs. The first used JELIS event rates (‘JELIS scenario’) and the second assumed higher rates of effectiveness for EPA-plus-statin therapy (‘alternate scenario’). Cost and utility values from the base case were applied to these two alternative clinical scenarios. Results were interpreted based on the joint ACC/AHA statement on cost/value methodologyCitation42.
Results
The results for the base case of the model are presented in . Under the base case conditions, over the 5-year period, costs were lower ($29,377 vs $30,587) and total utilities accrued were higher (3.627 vs 3.575) for patients treated with EPA-plus-statin therapy compared with statin monotherapy, demonstrating that the EPA-plus-statin therapy dominated. In this base case scenario, EPA was cost saving. There were consistently higher annual costs for pharmacologic therapy in the EPA group, but, by the third year, there were savings in total costs due to the increasing costs for treatment of MACCE in the statin monotherapy group. The cost savings occurred despite the fact that, in the statin monotherapy group, CV mortality is assumed to be higher and, thus, more people drop out entirely and accrue no further costs.
Table 4. Model results: base case.
presents results for sensitivity analyses that explore input parameters on rates and timing of events, patient utilities, costs of events, and overall assumptions (i.e. discount rate). In most scenarios, the results were not sensitive to multiple variations in model inputs and consistently identified EPA-plus-statin therapy to be the economically dominant strategy, with both lower costs and better patient utilities over the modeled 5-year period. Based on additional sensitivity analyses, EPA remained highly cost-effective (according to the framework of the ACC/AHA statement on cost/value methodologyCitation42) when event rates for the EPA + statin group were ∼70% of the rates in the statin group, and met the upper end of the threshold for intermediate care value only when rates were ∼78% of the statin group’s event rates. This threshold analysis takes into account only differences in event rates; similar analyses could be conducted with costs or utilities, but, as there is less uncertainty around these input parameters and their impact has less influence on model results, similar analyses have not been conducted around them.
Table 5. Modeled sensitivity analyses.
Discussion
The decision analytic model presented here compared the cost-effectiveness of EPA-plus-statin therapy with statin monotherapy under a set of base-case assumptions as well as under more extreme assumptions using sensitivity analyses. The findings of the model indicated that addition of EPA to statin therapy for secondary prevention of cardiovascular diseases consistently resulted in both cost savings and better patient outcomes as measured by utilities. The base case as well as most sensitivity analyses resulted in cost-effectiveness ratios that indicate high (to intermediate) care value according to the framework of the ACC/AHA cost/value methodologyCitation42.
The framework and initial clinical data source for model input for the EPA-plus-statin arm of this model was JELIS. In the full panel report of the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults, JELIS was evaluated as the only omega-3 fatty acid trial in the expert panel’s systematic reviewCitation20. In JELIS, EPA was co-administered with a statin and compared with statin monotherapy in both primary and secondary prevention populationsCitation3,Citation5. EPA 1,800 mg was added to statin therapy in a Japanese population of men and post-menopausal women with baseline LDL-C levels ≥170 mg/dL, with and without coronary heart disease. Compared with statin monotherapy, co-administration of EPA and statins did not reduce LDL-C, and reduced triglycerides only modestly (estimated difference of 5%). However, combined EPA and statin therapy reduced coronary heart disease events by 19% (HR = 0.81, 95% CI = 0.69–0.95, p = 0.011) compared with statin monotherapy in the intent-to-treat patient populationCitation3. Similar magnitudes of risk reduction were observed in primary and secondary prevention populationsCitation3,Citation5. However, JELIS was not powered for sub-group analysesCitation3. The ACC/AHA expert panel did not generalize the JELIS data to US populations due to the high dietary intake of omega-3 fatty acid, as fish intake is higher in the Japanese population compared with the USCitation3,Citation20, and potentially due to the low statin doses used in the JELIS trial relative to current statin doses commonly used in US populations. With regard to the statin dose, the doses of simvastatin and pravastatin used in the JELIS trial were based on Japanese guidelines and were supported by the CV risk reduction benefits shown with the same pravastatin dose in the MEGA trialCitation3,Citation43. The MEGA trial also included a Japanese population with a high dietary fish intake. However, it may be worth noting that the high plasma levels of EPA achieved by patients in the JELIS trial can also be achieved with a high-dose purified EPA in US patientsCitation28, and blood EPA levels may be associated with clinical significanceCitation44. An analysis of JELIS patients demonstrated that a higher on-treatment plasma level of EPA, but not DHA or other fatty acids, was significantly associated with a lower risk of MCE (HR = 0.71, 95% CI = 0.54–0.94, p = 0.018)Citation27.
This model utilized the framework of the JELIS secondary prevention trial to examine cost-effectiveness based on event rates in the EPA-plus-statin arm within JELIS compared with an event rate potentially more representative of the US population in the statin monotherapy arm based on event rates in the secondary prevention trials within the PROVE-IT-TIMI 22, TNT, and other CTT more vs less statin trialsCitation19,Citation22,Citation23. However, the model was designed to be dynamic and allow users such as managed care organizations to input event rates from the patient lives they cover to determine a tailored cost-effectiveness estimate. An analysis from a claims database looking at the prevalence and the results on outcomes with an omega-3 fatty acid plus statin regimen among patients discharged with MI in Italy showed a synergism between the effects of statins and omega-3 fatty acids for most CV outcomes, including all-cause mortality compared with statin monotherapy (incidence rate difference = −2.5 per 100 patients/year; 95% CI = −2.2 to −2.9, p < 0.001)Citation45. As this model evaluated the cost-effectiveness of high-purity EPA-plus-statin therapy, it should be noted that the results would only be expected for a high-purity prescription EPA product and should not be translated to other prescription omega-3 fatty acid products that contain DHA or to omega-3 fatty acid dietary supplements such as fish oilsCitation46–48. Costs may be different and DHA may raise LDL-CCitation13,Citation14,Citation17,Citation18, which may potentially alter the risks and incidences of CV events included in this model. Furthermore, the EPA and/or DHA content of dietary supplements may vary from the amount stated on the package label and notably, dietary supplements are not required by the US Food and Drug Administration to demonstrate clinical safety or efficacyCitation49.
Limitations
This model should be interpreted in the context of several important limitations. First, our model is only an approximation of a potential reality and is unable to capture all complexities of a real-world scenario. Many of the model inputs were derived from the literature and not from a single outcomes trial with a priori pharmacoeconomic end-points. The model allows for only a single event per year per patient and does not fully account for the possibility that patients could experience multiple events in a year or over time; there were no data to estimate such event rates or whether there were increases in costs or decreases in utilities associated with multiple events. Therefore, findings may under-estimate the cost-effectiveness of the EPA-plus-statin combination due to potential reductions in more than one event per patient. A recent IMPROVE-IT trial analysis showed a slightly greater relative risk reduction in total CV events (first and recurrent) compared with the first event onlyCitation50. The model reported herein was developed from the perspective of the US healthcare system, even though the EPA-plus-statin arm event rates were derived from the JELIS secondary prevention strata, a purely Japanese population. For the EPA-plus-statin arm, the model assumes that the event rates will be similar to the JELIS secondary prevention strata and this may not be the case in the US population. The JELIS secondary prevention strata were not representative of the US population with established CVD, as the rates of CVD in a secondary prevention population are higher in the US than in JapanCitation51,Citation52. However, because of this difference, and because the EPA plasma concentrations in JELIS (170 μg/mL) can be achieved with a high-purity EPA prescription product in the USCitation28,Citation29, one may expect the difference in event rates between statin monotherapy vs EPA-plus-statin therapy to be larger in the USCitation3,Citation27. The cost/quality-adjusted life year for the exact JELIS scenario in this report was not cost-effective for EPA. This may be a result of applying lower coronary event rates from a Japanese patient population, without adjustment, to a Western population inclusive of drug and intervention costs. The model is not unique in extrapolating Japanese event data to the USCitation20,Citation21. This model also excluded indirect costs and costs of adverse events and assumed sufficient adherence to medication; all of these approaches are consistent with previous cost-effectiveness analyses of lipid-modifying treatmentsCitation53. Additionally, the model is not meant to present a societal perspective and does not include non-medical or patient out-of-pocket costs. Based on our deterministic model design, an acceptability curve could not be derived.
Conclusions
This novel model suggests that combining EPA with statin therapy for secondary prevention of CVD in the US may be cost saving and appears to be a more compelling intervention than statin monotherapy. The robustness of this decision analytic model was confirmed by performing a range of sensitivity analyses by varying key inputs and assumptions. However, the treatment effect of icosapent ethyl (high-dose purified EPA) on CV outcomes remains to be proven; the results of the ongoing REDUCE-IT study (clinicaltrials.gov NCT01492361) utilizing icosapent ethyl with statin therapy will allow for a more refined cost-effectiveness microanalysis based on outcomes data in patients with persistent hypertriglyceridemia, including those from the US and other Western countriesCitation54.
Transparency
Declaration of funding
This study was designed and sponsored by Amarin Pharma Inc., Bedminster, NJ, USA.
Declaration of financial/other relationships
JKS and CKH-L are employed by Exponent, which received grant/research support from Amarin Pharma Inc. JN is on the speakers’ bureaus of Amarin Pharma Inc., Arbor, Amgen, Kowa Pharmaceuticals America, Inc., Merck, Regeneron, and Sanofi; is a consultant to Amarin Pharma Inc.; owns stock in Amarin Pharma Inc., Amgen, Pfizer, and Regeneron; and provides advisory services to Amarin Pharma Inc. SP and PBE are employees and stock shareholders of Amarin Pharma Inc. SC is a consultant to Amarin Pharma Inc. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial assistance was provided by Peloton Advantage, LLC, Parsippany, NJ, USA, and funded by Amarin Pharma Inc., Bedminster, NJ, USA.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29-322
- Fruchart JC, Davignon J, Hermans MP, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol 2014;13:26
- Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090-8
- Tanaka K, Ishikawa Y, Yokoyama M, et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke 2008;39:2052-8
- Matsuzaki M, Yokoyama M, Saito Y, et al. Incremental effects of eicosapentaenoic acid on cardiovascular events in statin-treated patients with coronary artery disease. Circ J 2009;73:1283-90
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-97
- Blaha MJ, Martin SS. How do statins work?: changing paradigms with implications for statin allocation. J Am Coll Cardiol 2013;62:2392-4
- Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047-67
- Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis 2015;242:357-66
- Tanaka N, Ishida T, Nagao M, et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis 2014;237:577-83
- Epadel [package insert, 2015 and pharmaceutical interview form, 2013]. Tokyo, Japan: Mochida Pharmaceutical Co., Ltd; 2015
- Vascepa [package insert]. Bedminster, NJ: Amarin Pharma Inc.; 2015
- Wei MY, Jacobson TA. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep 2011;13:474-83
- Jacobson TA, Glickstein SB, Rowe JD, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol 2012;6:5-18
- Bays HE, Ballantyne CM, Kastelein JJ, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol 2011;108:682-90
- Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol 2012;110:984-92
- Lovaza [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2013
- Epanova [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2014
- Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 report on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease in adults: full panel report supplement [online supplement]. Circulation 2014;129
- Guirguis-Blake JM, Evans CV, Senger CA, et al. Aspirin for the primary prevention of cardiovascular events: A systematic evidence review for the U.S. preventive services task force. Evidence synthesis No. 131. Rockville, MD : Agency for Healthcare Reseach and Quality; 2015
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495-504
- Magnuson EA, Farkouh ME, Fuster V, et al. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes mellitus and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013;127:820-31
- Cohen DJ, Osnabrugge RL, Magnuson EA, et al. Cost-effectiveness of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with 3-vessel or left main coronary artery disease: final results from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial. Circulation 2014;130:1146-57
- Dieleman JP, van Wyk JT, van Wijk MA, et al. Differences between statins on clinical endpoints: a population-based cohort study. Curr Med Res Opin 2005;21:1461-8
- Itakura H, Yokoyama M, Matsuzaki M, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb 2011;18:99-107
- Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med 2014;126:7-18
- Braeckman R, Bays HE, Ballantyne CM, et al. Pharmacokinetic and triglyceride-lowering pharmacodynamic effects of icosapent ethyl (eicosapentaenoic acid ethyl ester) across clinical studies [abstract 19343]. Circulation 2013;128:A19343
- Russell MW, Huse DM, Drowns S, et al. Direct medical costs of coronary artery disease in the United States. Am J Cardiol 1998;81:1110-15
- Wang G, Zhang Z, Ayala C, et al. Costs of hospitalizations with a primary diagnosis of acute myocardial infarction among patients aged 18–64 years in the United States. In: Gaze DC, editor. Ischemic heart disease. Rijeka, Croatia: InTech; 2013. p 77-90
- Azoulay A, Pilote L, Filion KB, et al. Costs of treatment of acute myocardial infarction in Canadian and US hospitals. Cardiovasc Rev Rep 2003;24
- Cowie MR, Cure S, Bianic F, et al. Cost-effectiveness of highly purified omega-3 polyunsaturated fatty acid ethyl esters in the treatment of chronic heart failure: results of Markov modelling in a UK setting. Eur J Heart Fail 2011;13:681-9
- Kongnakorn T, Ward A, Roberts CS, et al. Economic evaluation of atorvastatin for prevention of recurrent stroke based on the SPARCL trial. Value Health 2009;12:880-7
- Bloom BS, Tibi-Levy Y, Harari A, et al. Direct medical care costs of unstable angina pectoris in a defined population. J Managed Care Pharm 1999;5:39-44
- Osnabrugge RL, Speir AM, Head SJ, et al. Prediction of costs and length of stay in coronary artery bypass grafting. Ann Thorac Surg 2014;98:1286-93
- Brown PP, Kugelmass AD, Cohen DJ, et al. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg 2008;85:1980-6
- Eisenberg MJ, Filion KB, Azoulay A, et al. Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med 2005;165:1506-13
- Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA 2015;314:142-50
- Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology 2013;81:1588-95
- McConnachie A, Walker A, Robertson M, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J 2014;35:290-8
- Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 2014;129:2329-45
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006;368:1155-63
- Superko HR, Superko SM, Nasir K, et al. Omega-3 fatty acid blood levels: clinical significance and controversy. Circulation 2013;128:2154-61
- Macchia A, Romero M, D’Ettorre A, et al. Exploratory analysis on the use of statins with or without n-3 PUFA and major events in patients discharged for acute myocardial infarction: an observational retrospective study. PLoS One 2013;8:e62772
- The Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800-8
- ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309-18
- Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152-9
- Weintraub H. Update on marine omega-3 fatty acids: management of dyslipidemia and current omega-3 treatment options. Atherosclerosis 2013;230:381-9
- Giugliano RP, Cannon C, Blazing M, et al. Baseline LDL-C and clinical outcomes with addition of ezetimibe to statin in 18,144 patients post ACS [abstract]. J Am Coll Cardiol 2015;65:A4
- Kitamura A, Sato S, Kiyama M, et al. Trends in the incidence of coronary heart disease and stroke and their risk factors in Japan, 1964 to 2003: the Akita-Osaka study. J Am Coll Cardiol 2008;52:71-9
- Iso H. A Japanese health success story: trends in cardiovascular diseases, their risk factors, and the contribution of public health and personalized approaches. EPMA J 2011;2:49-57
- Michailov GV, Davies GM, Krobot KJ. Cost-effectiveness of extended-release niacin/laropiprant added to a stable simvastatin dose in secondary prevention patients not at cholesterol goal in Germany. Eur J Health Econ 2012;13:365-74
- A study of AMR101 to evaluate its ability to reduce cardiovascular events in high risk patients with hypertriglyceridemia and on statin (REDUCE-IT). http://clinicaltrials.gov/show/NCT01492361. Accessed June 20, 2016