Abstract
Objective: To evaluate the cost-effectiveness of exenatide twice daily (BID) vs bolus insulin lispro three times daily (TID) as add-on therapy when glycemic control is sub-optimal with titrated basal insulin glargine and metformin.
Methods: The analysis was based on the recent 4B Study, which compared exenatide BID and lispro TID as add-on therapies in subjects with type 2 diabetes insufficiently controlled, despite titrated insulin glargine. The Cardiff Diabetes Model was used to simulate patient costs and health benefits beyond the 4B Study. The Swedish healthcare perspective was adopted for this analysis; costs are reported in €EUR to aid interpretation. The main outcome measure was the cost per quality-adjusted life-year (QALY) gained with exenatide BID compared to lispro TID.
Results: Exenatide BID was associated with an incremental cost of €1,270 and a QALY increase of +0.64 compared with lispro TID over 40 years. The cost per QALY gained with exenatide BID compared with lispro TID was €1,971, which is within conventional limits of cost-effectiveness. Cost-effectiveness results were generally robust to alternative assumptions and values for key model parameters.
Limitations: Extrapolation of trial data over the longer term can be influenced by modeling and parameter uncertainty. Cost-effectiveness results were generally insensitive to alternative values of key model input parameters and across scenarios.
Conclusions: The addition of exenatide BID rather than insulin lispro to basal insulin is associated with similar or better clinical outcomes. Illustrated from the Swedish healthcare perspective, analysis with the Cardiff Diabetes Model demonstrated that exenatide BID represents a cost-effective treatment alternative to lispro TID as add-on therapy in type 2 diabetes patients insufficiently controlled on basal insulin.
Introduction
The overall aim of treatment in type 2 diabetes is the concomitant management of glycemic control, weight gain, and hypoglycemia; however there are typically trade-offs between treatment options in achieving one or a composite of these endpointsCitation1. These trade-offs are known to vary according to patient phenotype, prior treatment history, and disease status.
The focus of this research is on patients with advanced disease with limited therapeutic options. One of the therapeutic approaches available to type 2 diabetes patients insufficiently controlled on basal insulin is to add bolus insulin. Whilst this approach has been shown to lower glycosylated hemoglobin (HbA1c), the trade-off is commonly weight gain and an increased incidence of hypoglycemic episodesCitation1. An alternative treatment strategy to the addition of bolus insulin is the use of the GLP-1 receptor agonist exenatide twice daily (BID). This was evaluated in the 4B Study, a randomized clinical trial of patients with type 2 diabetes and inadequate glycemic control, pre-treated with optimized basal insulin glargine in combination with metforminCitation2. Based in part on evidence from this study, the addition of a GLP-1 receptor agonist is now considered an option in those patients on basal insulin with one or more oral agents whose diabetes remains uncontrolledCitation3.
In choosing the most appropriate add-on treatment for patients with uncontrolled diabetes, insulin-associated weight gain is a key consideration as it can be disabling in patients with type 2 diabetes, potentially adversely affecting cardiovascular riskCitation4. Weight gain and hypoglycemia are also associated with indirect consequences; notably each can be a barrier to treatment satisfaction and compliance. Studies have shown that lower treatment satisfaction can lead to reduced treatment compliance, and that these factors can reduce treatment effectivenessCitation5.
In addition to evidence concerning treatment effectiveness, an accurate estimation of the comparative costs and cost-effectiveness of type 2 diabetes therapies may help to inform treatment decisions and algorithms in the future. Improving understanding and dissemination of the economic consequences of therapy choice among healthcare providers may, therefore, help to support complex clinical decisions and define favorable treatment practices based on potential benefits, risks, and costs.
The aim of this study was to undertake an applied economic evaluation to estimate the cost-effectiveness of exenatide BID vs insulin lispro TID as add-on treatment options based on the 4B Study results. We illustrate the costs and benefits in the Swedish healthcare setting.
Patients and methods
Cost-effectiveness model
The Cardiff Model, a stochastic simulation model, was utilized for this evaluationCitation6. The model is designed to evaluate the impact of therapies in a population of type 2 diabetes patients, modeling disease progression through implementation of the UK Prospective Diabetes Study (UKPDS) 68 outcomes equationsCitation7. The model has been the basis of a number of applied evaluations of type 2 diabetes therapiesCitation8,Citation9, and has been subject to peer-reviewed validationCitation10.
The model required specification of age, sex, ethnicity, smoking status, and duration of type 2 diabetes and model changes to modifiable risk factors: HbA1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic blood pressure, and weight. Time-dependent risk factor profiles were simulated through implementation of equations reported in the UKPDS 68 studyCitation7. On entering the simulation, patients were initialized to the selected therapy. Following the modification of each patient’s HbA1c in line with treatment effect, the model projected HbA1c over time. Pre-specified HbA1c threshold values were used to invoke escalation to the next line of therapy. Costs were applied to all predicted complications in the year of occurrence. Healthcare maintenance costs were applied in all subsequent years following non-fatal events. Baseline health state utility was modeled using age-dependent mean EQ-5D values (a standardized measure of health outcome) in subjects with no major complications, obtained from the Health Survey for England 2003Citation11. The occurrence of diabetes-related complications is associated with reductions in health-related quality-of-life, which were applied through utility decrements.
Model output included the incidence of microvascular (blindness, nephropathy, amputation) and macrovascular complications (congestive heart failure, myocardial infarction, stroke, ischemic heart disease), hypoglycemia, diabetes-specific mortality and all-cause mortality, point estimates, and probabilistic output for cost-effectiveness. Mortality relating to cardiovascular disorders and diabetes has already been accounted for in the above risk equations. Therefore, cardiovascular and diabetes-related deaths were subtracted from the all-cause mortality. Swedish-specific mortality rates were used.
The model was run over a 40-year time horizon (lifetime perspective) in half-year cycles. Costs (€EUR) and benefits were discounted at a rate of 3% per year. The chosen measure of health benefit was the quality-adjusted life-year (QALY). Cost-effectiveness was expressed as the cost per QALY gained with exenatide BID compared with lispro TID.
Base case analysis
The base case analysis considered the use of exenatide BID in patients with type 2 diabetes inadequately controlled on insulin glargine therapy in combination with metformin, as an alternative to the addition of lispro TID. Baseline characteristics for the economic analysis were sourced from the 4B Study and are presented in full in . The 4B Study was a randomized clinical trial of 627 patients with type 2 diabetes and inadequate glycemic control (HbA1c >7.0%, or >53 mmol/mol), pre-treated with optimized basal insulin glargine in combination with metformin. The study compared the efficacy and safety of exenatide BID to mealtime insulin lispro TID over 30 weeks, amongst inadequately controlled patients. Patients had a 12-week run-in period to optimize basal insulin dose to exclude patients who could reach goal with basal insulin alone. Patients were concomitantly treated with basal insulin glargine and metformin in the randomization periodCitation2. Characteristics at randomization were similar across arms, with patients having a mean age of 59.8 years, mean HbA1c of 8.2% (66 mmol/mol), and median diabetes duration of 12 yearsCitation2.
Table 1. Data inputs: Baseline characteristics and treatment effects (mean, SETable Footnote*).
HbA1c progression
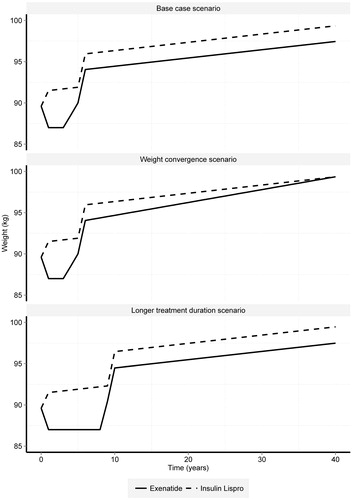
The HbA1c effects observed in the 4B Study were applied to baseline HbA1c in the first year of the model (). HbA1c progression was modeled as a gradual increase following the applied treatment effect (year 1) to the time of therapy escalation (estimated in the model to occur when HbA1c reaches its baseline value; year 4.5). Following therapy escalation, HbA1c was modeled to gradually increase over the remainder of the time horizon (for the base case and longer duration scenarios)Citation7.
Rescue therapy was modeled as a switch to intensified basal-bolus therapy based on the study by Rosenstock et al.Citation12, where patients previously treated with insulin glargine plus oral agents required escalation of insulin therapy. The HbA1c treatment effect applied in the model at the start of rescue therapy was made proportional to the difference in HbA1c at the time of switch (8.20%; 66 mmol/mol) and the baseline value observed in the Rosenstock et al.Citation12 study (8.90%) and is calculated to be −1.93% [(8.20/8.90) × −2.09%].
Weight progression
A weight treatment effect was applied to each arm in the first year of treatment. Weight change over the first 4.5 years of treatment was modeled differently for the exenatide BID and lispro TID arms. For the exenatide BID arm, weight reduction was assumed to apply for the period a patient remains on therapy (4.5 years in the base case analysis), which itself was determined by the time to rescue therapy. The treatment effect was applied in the first year and maintained over the first 3.5 years of treatment, and weight returned to its baseline value over years 3.5–4.5 (including the assumed effect of natural weight gain, 0.1 kg/year). For the lispro TID group, weight gain was applied over the first year of treatment, and natural weight (0.1 kg/year) is assumed for the remainder of time on therapy prior to switch. The weight treatment effect reported in the study by Rosenstock et al.Citation12 (+4.50 kg) was made proportional to the model’s estimate of average weight across each model arm at the time of switch (90.90 kg) and the baseline weight profile reported by Rosenstock et al.Citation12 (99.80 kg), calculated as 4.10 kg [(90.90/99.80) × 4.50]. After applying the treatment effect associated with rescue therapy, an annual 0.1 kg natural weight gain was applied in each year of the remaining modeled time horizon.
Systolic blood pressure and cholesterol treatment effects
Treatment effects for systolic blood pressure (SBP), total, and HDL cholesterol were applied to each arm in the first year of treatment ().
Healthcare costs
The model considered direct medical costs for medications, consumables (i.e. test strips, lancets, and needles), and type 2 diabetes-related complications (including discontinuations and episodes of severe hypoglycemia) (). All costs were sourced with the Swedish healthcare perspective and expressed in 2015 SEK using the Swedish Consumer Price Index (CPI)Citation13. Conversion into €EUR was undertaken using the rate of exchange in 2015Citation14 of SEK:EUR (0.108).
Table 2. Data inputs: Annual cost of medications and complications (€EUR) and health state utility changes.
Medications
Cost of medications were based on current unit pricesCitation15. Medication costs for exenatide BID and lispro TID were based on usage during the 4B StudyCitation2. The insulin requirement at the time of switch to rescue therapy is a function of patient weight. Hence, adjusted insulin units per kg per day (IU/kg/day) was calculated [(a) × (b), see below] to be proportional to the difference between the model’s estimate of average weight across each model arm at the time of switch and the baseline weight profile reported in the Rosenstock et al.Citation12 study [(90.90/99.80), (a)], and the end insulin doses reported in the Rosenstock et al.Citation12 study (b). This adjustment ensures the absolute costs associated with insulin use and rescue therapy were accurate; the same daily insulin requirement (IU/kg/day) was applied to each arm of the model, from which the total annual insulin cost associated with rescue therapy was calculated ().
Consumables
Consumable costs were applied to each arm as the sum of costs for test strips, lancets, and needles. Insulin glargine therapy was assumed to include the use of one self-monitoring of blood glucose (SMBG) test strip per day, one lancet for SMBG, and one needle per day. In addition to these, lispro TID therapy was assumed to include the use of three SMBG test strips per day, three lancets for SMBG, and three needles per day. Exenatide BID was assumed to include an additional two needles per day, but no additional SMBG requirement above that of insulin glargine. The cost per unit for test strips, lancets, and needles were €0.46, €0.04, and €0.13, respectively ()Citation16.
Complications
The costs of type 2 diabetes-related complications were extracted from a Swedish study by Gerdtham et al.Citation17 using administrative hospitalization data. For fatal congestive heart failure and amputation events, the cost of a non-fatal event was assumed. Further, event costs in “subsequent years” were used to characterize annual maintenance costs in the model.
The cost associated with the occurrence of blindness was sourced from the study by Henriksson et al.Citation18. Maintenance costs were characterized as the costs associated with “annual follow-up after first 12 months”.
Regarding hypoglycemia, only the cost of moderate hypoglycemic events was included in the model. The cost for moderate hypoglycemia obtained from a Swedish studyCitation19 was used as it most closely reflected the definition of severe hypoglycemia used in the Cardiff model, the 4B Study and the Rosenstock et al.Citation12 study.
Finally, drug discontinuations due to adverse events (AEs) that occurred in the first 6 months were attached a standard cost equal to the cost of a visit to a general practitioner (Supplementary Information; Table S1).
Health state utility data
The health state utility decrements associated with age, type 2 diabetes-related complications, and hypoglycemia are reported in .
The UKPDS 62 data were used to characterize the impact of type 2 diabetes-related complications on health-related quality-of-life. Utility decrements associated with episodes of hypoglycemia were extracted from the study by Currie et al.Citation20; equations characterizing the relationship between the fear of hypoglycemia and health-related utility using the EQ-5D were hard coded into the model. The utility change associated with a one-unit change in body mass index (BMI) was sourced from Lane et al.Citation21.
Treatment-related adverse events
A discontinuation rate due to AEs was applied to each arm of the model, based on data from the ITT population of the 4B StudyCitation2. The same study was used to characterize the number and probability of hypoglycemia episodes. For rescue therapy, numbers were based on data reported in Rosenstock et al.Citation12.
Sensitivity analyses
Deterministic sensitivity analyses
A number of one-way sensitivity analyses were undertaken to explore the impact of alternative parameter values for key model inputs on estimates of cost-effectiveness: variation in discount rate (discounting 0 to 6%); variation in time horizon (20 years); variation of complication costs (± 20% mean value); variation of complication disutility (± 20% mean value); variation in the utility change associated with a one-unit change in BMI (a 0.006 utility change associated with a one-unit change in BMICitation22); variation in the utility change associated with a one-unit change in BMI (a 0.014 utility change associated with a one-unit change in BMICitation23).
HbA1c reduction during the 4B Study was −1.10% in the exenatide group and −1.07% in the lispro TID group (ITT population)Citation2. Based on the demonstration of non-inferiority during the 4B Study, HbA1c treatment effects for exenatide BID and lispro TID treatment arms were considered the same (−1.10%) for the base case analysis; hence, values for these parameters were not assessed in one-way sensitivity analysis. However, the impact of an alternative weight progression profile was explored in the following model analyses for each successive year ().
Duration of treatment. The base case analysis evaluated a switch to rescue therapy after ∼4.5 years of treatment based on a return to baseline HbA1c of 8.20% (66 mmol/mol). An alternative switching rule was considered where the switch to rescue therapy occurs at 8.90%, corresponding to average treatment duration of 9 years. The HbA1c and weight treatment effects were then applied as reported in Rosenstock et al.Citation12. The same assumptions were made; the treatment effect associated with the exenatide BID arm is maintained over the duration of therapy (treatment reduction in year 1, weight effect maintained to year 8, and a gradual return to baseline weight from years 8–9); and the lispro TID arm experiences weight gain over the first year of treatment, and natural weight (0.1 kg/year) is assumed for the remainder of time on therapy prior to switch.
After applying the treatment effect associated with rescue therapy, an annual 0.1 kg natural weight gain is applied in each year of the remaining modeled time horizon for each arm ().
Weight convergence. An alternative weight progression profile was considered for the periods following switch to rescue therapy (year 4.5+). This analysis allowed the weight curves describing weight progression in the exenatide BID and lispro TID arms to converge over time such that the difference is reduced.
Probabilistic sensitivity analyses
The impact of the joint uncertainty in the model parameters was explored in a probabilistic sensitivity analysis of the model. A non-parametric bootstrapping approach was used to sample from distributions around the means of input parameters in the model. Cohort characteristics and treatment effects were based on means and standard errors reported in the 4B Study or the source referenced in the base case analysis. Complication costs and utility decrements were based on variation of ±10% of mean value. The results of these analyses are presented as incremental cost-effectiveness ratio (ICER) scatterplots, illustrating the distribution of cost and effect pairs for each scenario, and cost-effectiveness acceptability curves (CEAC), illustrating the proportion of the cost and effect pairs where the intervention is cost-effective for a given willingness to pay per QALY threshold.
Results
Base case cost-effectiveness
In the base case analysis, exenatide BID was associated with an incremental cost of €1,270 and an incremental QALY of +0.64 over 40 years. The cost per QALY gained with exenatide BID compared with lispro TID was €1,971 ().
Table 3. Expected costs, QALYs, and incremental cost-effectiveness (1000 people over 40 years): base case and deterministic sensitivity analysis (1000 people over 40 years).
Sensitivity analysis
Deterministic sensitivity analyses
Results of the deterministic sensitivity analyses are reported in . Across analyses, the ICER ranged from €1,344–€8,076. Model estimates were relatively insensitive to individual changes in parameter values for discounting, complication costs, and disutility, holding all else constant. Model estimates were most sensitive to the utility change associated with a one-unit change in BMI.
Probabilistic sensitivity analyses
Results of the probabilistic sensitivity analyses are reported in Supplementary Information Figures S1 and S2. In this analysis, the exenatide BID arm generated a mean QALY gain of 0.75 over a patient’s life-time, at an average incremental cost of €1,102. These estimates correspond to a mean cost per QALY-gained of €1,471 with exenatide BID compared to lispro TID.
The ICER scatterplot (Supplementary Information; Figure S1) illustrates that the distribution of cost and effect pairs from the probabilistic analysis were primarily in the upper right (North East) quadrant of the cost-effectiveness plane; this suggests that exenatide BID provides additional benefit compared to lispro TID at higher cost when used as an add-on treatment to basal insulin in the context of this analysis. The CEAC (Supplementary Information; Figure S2) from this analysis suggests the probability that the exenatide BID arm is cost-effective compared to the lispro TID arm is very high, even at willingness-to-pay thresholds of €5,000, €25,000, and €50,000 (99.9%, 100%, and 100%, respectively), which is considerably below conventional limits of cost-effectiveness: in the Swedish context, no formal willingness-to-pay threshold exists, but interventions less than 500,000 SEK (€54,000) per year QALY-gained may be considered cost-effectiveCitation24.
Discussion
Informed clinical decisions require the consideration of complex and inter-related factors including patient socioeconomic and medical history, expected therapeutic response to treatment, and the costs associated with treatment. Health outcomes and costs can occur in both the short and longer terms, and, whilst practicing clinicians may not be able to directly observe these consequences, health outcomes and costs are, nonetheless, relevant consequences that follow from treatment decisions. For example, total costs and outcomes of diabetes management will depend on the type of patient and the choice and frequency of use of treatments; in this context, the health and economic consequences of different approaches to patient management included in treatment guidelines may be of particular interest to clinicians. We, therefore, undertook an analysis using an established diabetes model to support complex clinical decisions and define favorable treatment practices based on potential benefits, risks and costs, in patients uncontrolled on basal insulin who have limited therapeutic options. Patients treated with exenatide BID were compared to the strategy of adding bolus insulin for uncontrolled diabetes. The analysis was based on the results of the 4B Study that demonstrated similar glycemic control with less hypoglycemia with exenatide BID when compared with lispro TID. Furthermore, exenatide BID was associated with a weight loss of 2.6 kg and lispro TID with a weight gain of 1.9 kg (ITT population)Citation2.
Over the projected lifetime of patients, the exenatide BID arm was associated with more health benefits (0.64 additional QALYs). The QALY gains associated with exenatide BID were driven by the superior weight profile associated with exenatide BID vs lispro TID regimens over the modeled time horizon and the avoidance of the disutility associated with weight gain. The incremental cost per QALY-gained estimate was €1,971, which is within conventional limits of cost-effectiveness in Sweden as well as in other European countries and the US. The cost-effectiveness of exenatide BID based on the 4B Study has also been evaluated in the Spanish and Turkish healthcare settings, where exenatide BID was predicted to be a cost-effective alternative to lispro TIDCitation25,Citation26.
The sensitivity of estimated cost-effectiveness was evaluated for alternative weight profiles for exenatide BID and lispro TID. Where the weight progression benefit associated with the exenatide BID arm was reduced by allowing weight to converge between the two groups over time, cost-effectiveness was reduced from €1,971 to €2,568 per QALY-gained, but remained well within conventional limits of cost-effectiveness. In the scenario where treatment duration with exenatide BID and lispro TID regimens was increased from 4.5 to 9 years, the estimated cost-effectiveness ratio increased from €1,971 to €3,343 due to a longer duration on these regimens.
Cost-effectiveness results were generally insensitive to alternative values of key model input parameters. Model estimates were most sensitive to the change in utility associated with changes in BMI. Nonetheless, the ICERs were all within acceptable limits of cost-effectiveness for all modeled BMI-related utility values. In the probabilistic sensitivity analysis the probability that the exenatide BID arm is cost-effective compared to the lispro TID arm was estimated to be 99.9% at a willingness-to-pay threshold of €5,000 and 100% at €50,000. Hence, the base case result that exenatide BID is a cost-effective treatment alternative to lispro TID as an add-on to basal insulin was robust to alternative assumptions and values for key model parameters. Nonetheless, there remain areas of uncertainty that may impact estimated cost-effectiveness. The Cardiff model uses the UK Prospective Diabetes Study (UKPDS) 68 outcomes equations to predict disease progression through type 2 diabetesCitation7, similar to other widely used models of type 2 diabetesCitation10. Although BMI and weight are associated with increased risk of complications and mortality in patients with type 2 diabetesCitation27, BMI is only identified as a risk factor in one of the equations used to estimate the probability of diabetes-related complications (congestive heart failure)Citation7. Thus, models of disease progression using these equations may under-estimate the impact of reduced risk of complications from treatment strategies with a proven beneficial effect on weight in terms of reduced healthcare resource utilization and improved quality-of-life.
Further, the pre-specified HbA1c threshold used to invoke escalation of therapy within this study is higher than that typically recommended by international guidelines on diabetes managementCitation28,Citation29. There is a documented disconnect between guidelines and clinical practice; one UK-based study reported a mean HbA1c level of 9.4% in patients with type 2 diabetes intensified with insulin, while a systematic review reported that mean HbA1c at insulin intensification ranged from 7.52–10.7% in identified studiesCitation30,Citation31. The HbA1c threshold utilized within this study is well within this range. Even though the HbA1c threshold in this study was above that recommended by guidelines, model sensitivity analysis based on alternative HbA1c thresholds did not significantly impact the estimated cost-effectiveness of exenatide BID, as seen in .
The analysis conducted represents a long-term evaluation of costs and effects using a simulation model that has been consistently used in comparable cost-effectiveness analyses of exenatide BID vs insulin across Europe, including Spain and TurkeyCitation25,Citation26. Results of these studies, which apply the same methodology (i.e. use of UKPDS equations, 3% discounting and healthcare payer perspective) support the result presented herein; ICERs with high probability of cost-effectiveness (near 100%) were predicted across studies, with similar QALY gains estimatedCitation25,Citation26. The use of direct medical costs only could be considered a limitation; however, this approach was selected to be transparent and would be considered conservativeCitation32. Another potential limitation of the study is the use of utilities that were not collected alongside the pivotal trial, and are, therefore, not as contemporary as the 4B Study data, and are from the UK. This may raise queries around the generalizability of these inputs to the Swedish population; however, a single source for Swedish-specific inputs for all of the diabetes-related events evaluated could not be identified; conversely, the UKPDS utilities have been estimated with a consistent methodology, which makes these utilities comparable across different diabetes-related events. Finally, the inputs underlying the presented cost-effectiveness analysis were based on the findings of the 4B Study, a randomized clinical trial of patients with type 2 diabetes and inadequate glycemic control, pre-treated with optimized basal insulin glargine in combination with metforminCitation2; a systematic review or network meta-analysis of different treatment options for patients who are uncontrolled on basal insulin would be an interesting topic for future research, which is beyond the scope of the objectives of this study.
Conclusion
In conclusion, the practice of adding bolus insulin when glycemic control is inadequate with basal insulin is associated with weight gain and an increased incidence of hypoglycemia, and may be viewed as sub-optimal from the patient and provider perspectives. The use of exenatide BID as a treatment alternative to bolus insulin has been associated with similar glycemic control, whilst reducing patient body weight and the incidence of hypoglycemia. The addition of a GLP-1 receptor agonist is considered an option in patients not achieving optimal glycemic control on basal insulin with one or more oral agentsCitation3. With a base case estimated ICER of €1,971, evaluated against commonly used cost-effectiveness threshold values, exenatide BID represents a cost-effective treatment alternative to lispro TID as add-on therapy to titrated insulin glargine in patients with type 2 diabetes.
Transparency
Declaration of funding
AstraZeneca funded the study and preparation of this manuscript.
Declaration of financial/other relationships
JG and PM are employees of HEOR Ltd. US and BK are employees of AstraZenenca. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary figure 2
Download TIFF Image (115.3 KB)Supplementary figure 1
Download TIFF Image (107.3 KB)Supplementary table
Download MS Word (17.1 KB)Acknowledgments
We acknowledge writing support from Dr Daniel Sugrue and Beverley Jones (HEOR Ltd). The study authors extend our thanks to the participating research physicians, nurses, and patients who made this study possible.
References
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide-1 receptor agonist or bolus insulin with optimized basal insulin in diabetes. Diabetes Care 2014;37:2763-73
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9
- Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab 2007;9:799-812
- Pollack MF, Purayidathil FW, Bolge SC, et al. Patient-reported tolerability issues with oral antidiabetic agents: associations with adherence; treatment satisfaction and health-related quality of life. Diabetes Res Clin Prac 2010;87:204-10
- McEwan P, Peters JR, Bergenheim K, et al. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster). Curr Med Res Opin 2006;22:121-9.
- Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- Granström O, Bergenheim K, McEwan P, et al. Cost-effectiveness of saxagliptin (Onglyza®) in type 2 diabetes in Sweden. Prim Care Diabetes 2012;6:127-36
- van Haalen HGM, Pompen M, Bergenheim K, et al. Cost-effectiveness of dapagliflozin as add-on to insulin for the treatment of type 2 diabetes in the Netherlands. Value Health 2013;16:A441
- Palmer AJ, Clarke P, Gray A, et al. Computer modeling of diabetes and its complications: a report on the Fifth Mount Hood challenge meeting. Value Health J Int Soc Pharmacoeconom Outcomes Res 2013;16:670-85
- Department of Health. Health Survey for England; 2003. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/publicationsandstatistics/publications/publicationsstatistics/dh_4098712. Accessed September 15, 2014
- Rosenstock J, Ahmann AJ, Colon G, et al. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008;31:20-5
- Statistics Sweden (SCB). Swedish consumer price index (health component). 2015. http://www.scb.se/en_/Finding-statistics/Statistics-by-subject-area/Prices-and-Consumption/Consumer-Price-Index/Consumer-Price-Index-CPI/Aktuell-Pong/33779/Consumer-Price-Index-CPI/33907/. Accessed September 15, 2015
- Federal Reserve. Foreign Exchange Rates - G.5A Annual. 2014. http://www.federalreserve.gov/releases/G5a/current/default.htm. Accessed September 15, 2015
- Dental and Pharmaceutical Benefits Agency. Medication price list. 2015. http://www.tlv se/. Accessed November 17, 2015
- Apoteket AB. Medication price list. 2015. http://www.apoteket.se. Accessed November 17, 2015
- Gerdtham UG, Clarke P, Hayes A, et al. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. Pharmacoeconomics 2009;27:81-90
- Henriksson F, Agardh CD, Berne C, et al. Direct medical costs for patients with type 2 diabetes in Sweden. J Intern Med 2000;248:387-96
- Jönsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with type 2 diabetes in Sweden. Value Health 2006;9:193-8
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Lane S, Levy AR, Mukherjee J, et al. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin 2014;30:1267-73
- Bagust A, Beale S. Modelling EuroQol health‐related utility values for diabetic complications from CODE‐2 data. Health Econ 2005;14:217-30
- Jaime Caro J, Ozer Stillman I, Danel A, et al. Cost effectiveness of rimonabant use in patients at increased cardiometabolic risk: estimates from a Markov model. J Med Econ 2007;10:239-54
- Kiadaliri AA, Gerdtham UG, Eliasson B, et al. Cost-utility analysis of glucagon-like Peptide-1 agonists compared with dipeptidyl peptidase-4 inhibitors or neutral protamine hagedorn Basal insulin as add-on to metformin in type 2 diabetes in sweden. Diabetes Ther Res Treat Educ Diabetes Relat Dis 2014;5:591-607
- Malhan S, Güler S, Yetkin I, et al. Cost-effectiveness of exenatide twice daily (bid) added to basal insulin compared to a bolus insulin add-on in Turkey. Proceedings of the ISPOR 17th Annual European Congress, Vol. 17, A349. Value in Health, Amsterdam, The Netherlands, November 8–12, 2014
- Sánchez-Covisa J, Capel M, Baeten S, et al. Comparative cost-effectiveness analysis of adding twice-daily exenatide to insulin glargine versus adding insulin lispro to treat type 2 diabetes in Spain. Proceedings of the ISPOR 17th Annual European Congress, Vol. 17, A349–A350. Value in Health, Amsterdam, The Netherlands, November 8–12, 2014
- Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009;52:65-73
- National Institute for Health and Care Excellence. Clinical Guideline 87. Type 2 diabetes: The management of type 2 diabetes. 2009. http://www.nice.org.uk/Guidance/CG87. Accessed September 15, 2014
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364-79
- Asche CV, Bode B, Busk AK, et al. The economic and clinical benefits of adequate insulin initiation and intensification in people with type 2 diabetes mellitus. Diabetes Obes Metab 2012;14:47-57
- Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411-17
- Sabale U, Ekman M, Granstrom O, et al. Cost-effectiveness of dapagliflozin (Forxiga(R)) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries. Prim Care Diabetes 2015;9:39-47.
- Bristol-Myers Squibb Pharmaceuticals Ltd & Eli Lilly and Company. Final Clinical Study Report: H8O-EW-GWDM. A randomized trial comparing two therapies: Basal insulin glargine, exenatide and metformin therapy (BET) or basal insulin glargine, bolus insulin lispro and metformin therapy (BBT) in subjects with type 2 diabetes who were previously treated by basal insulin glargine with either metformin or metformin and sulfonylurea (4B: basal insulin glargine, exenatide BID, and metformin therapy or basal insulin glargine, bolus insulin lispro and metformin therapy). Indianapolis (Indiana, USA); 2013
- Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769-818
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65