Abstract
Objectives: Non-adherence and non-persistence to anti-hyperglycemic agents are associated with worse clinical and economic outcomes in patients with type 2 diabetes. This study evaluated treatment persistence and adherence across newer anti-hyperglycemic agents (canagliflozin, dapagliflozin, sitagliptin, saxagliptin, linagliptin, liraglutide, or exenatide).
Methods: This retrospective cohort study of Truven Health Analytics Marketscan databases included adult patients with type 2 diabetes whose first pharmacy claim for a newer anti-hyperglycemic agent was between February 1, 2014 and July 31, 2014. Treatment persistence and adherence were assessed for 12 months after the first claim (post-index). Persistence was defined as no gap ≥90 days between the end of one pharmacy claim and the start of the next pharmacy claim post-index. Adherence used two definitions: proportion of days covered (PDC) and medication possession ratio (MPR). Multivariable analyses of non-persistence (hazard ratios) and adherence (odds ratios) were adjusted for baseline demographics, drug cost, clinical characteristics, and other anti-hyperglycemic agents.
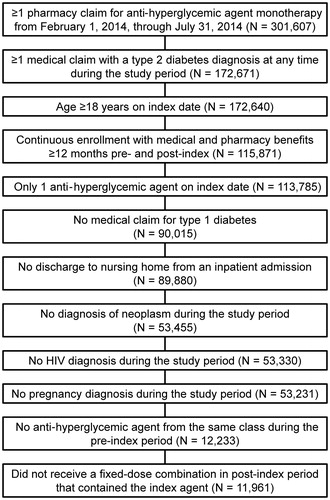
Results: A total of 11,961 patients met all study selection criteria. Persistence rates at 12 months were significantly greater (p < 0.05 for each comparison) for canagliflozin 100 mg (61%) compared with dapagliflozin 5 mg (40%), dapagliflozin 10 mg (41%), sitagliptin (48%), saxagliptin (42%), linagliptin (52%), liraglutide (47%), exenatide (23%), and long-acting exenatide (39%). The persistence rate was greater (p < 0.05) for canagliflozin 300 mg (64%) vs canagliflozin 100 mg. Median adherence rates for canagliflozin 100 mg (MPR = 0.83; PDC = 0.79) and canagliflozin 300 mg (MPR = 0.92; PDC = 0.81) were greater than for the other index anti-hyperglycemic agents (MPR = 0.33–0.75; PDC = 0.33–0.72). Consistent results for treatment persistence and adherence were observed in multivariable analyses that were adjusted baseline characteristics.
Conclusions: Canagliflozin was associated with better treatment persistence and treatment adherence compared with other anti-hyperglycemic agents in real-world settings.
Introduction
Treatment persistence and treatment adherence are similar, yet distinct, measurements of the degree to which a patient continues a treatment after initiationCitation1. Persistence refers to continuation of therapy, which is measured in claims-based analyses as the time until treatment discontinuation or a substantial gap in refills. Adherence refers to how often the patient takes the drug as prescribed, which is usually measured either by a proportion of days covered (PDC) that calculates the percentage of days in a given period that were covered by pharmacy claims, or by a medication possession ratio (MPR) that calculates the total days of supply by adding up all claims within the given period.
A recent study of 228,846 diabetes patients determined that only 46% had good (i.e. >80%) treatment adherence to oral anti-hyperglycemic agentsCitation2. In a meta-analysis of 34 studies of patients with type 2 diabetes mellitus who received oral anti-hyperglycemic agents in real-world settings, persistence to the index medication was ∼49% overall and ranged from 33–61% in the individual studiesCitation3. The same meta-analysis examined adherence for oral anti-hyperglycemic agents and reported that the pooled mean MPR was 75% and the proportion of patients with good adherence (≥80% MPR) was 68% overall; however, the mean MPR in individual studies ranged from 52–88%, and the rate of good adherence in individual studies ranged from 44–90%Citation3. Thus, it is important to examine treatment adherence rates for individual anti-hyperglycemic agents separately.
Multivariable analyses have reported that younger ageCitation4, lower socioeconomic statusCitation4, female genderCitation4,Citation5, and receiving medical care in the Southern USCitation4,Citation6 are predictors for worse treatment adherence. Non-adherence and non-persistence with anti-hyperglycemic agents have been associated with worse clinical and economic outcomes including poorer hemoglobin A1c (HbA1c) control, greater hospitalization rates, higher mortality rates, and higher healthcare costsCitation2,Citation7–13. Thus, the consequences of poorer treatment adherence and persistence are in direct contrast to the triple aim of clinical care proposed by the Institute for Healthcare Improvement: improved patient experience of care (including quality and satisfaction); improved health of populations; and reduced per capita costCitation14,Citation15. To address these issues, adherence to diabetes medications has been proposed as a quality measure for health plansCitation16, endorsed by the National Quality ForumCitation17, and adopted as a quality measure in the Centers for Medicare & Medicaid Services (CMS) star system for Medicare Part D plan ratingsCitation18. Physicians under the Medicare Physician Fee Schedule are now subjected to differential payment based on quality of care. Although adherence rates are not used to determine physician payments directly, total per capita cost for patients with diabetes is a value-based payment modifier and total costs are higher in patients with diabetes who are non-adherent to treatmentCitation11–13.
Intensive glycemic control has been shown to reduce the complications of diabetes, and treatment guidelines for pharmacologic therapy for type 2 diabetes recommend metformin as the initial monotherapyCitation19. If the HbA1c target is not achieved after ∼3 months, then combination therapy is recommended with metformin and any one of six classes of medications. The three classes of older anti-hyperglycemic agents include sulfonylureas, thiazolidinediones, and insulin. The three newer classes of anti-hyperglycemic agents approved for use either as monotherapy or in combination with other anti-hyperglycemic agents include: sodium/glucose cotransporter 2 (SGLT2) inhibitors such as canagliflozin, dapagliflozin, and empagliflozin; dipeptidyl peptidase-4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, linagliptin, and alogliptin; and glucagon-like peptide-1 (GLP-1) receptor agonists such as liraglutide, exenatide, albiglutide, and dulaglutide.
A few studies have evaluated treatment adherence between newer anti-hyperglycemic agents, but those studies only compared selected agentsCitation20–24. Differences in efficacy, tolerability, associated patient experiences, and cost, including patient out-of-pocket costs, may contribute to differences in treatment persistence and adherence not only between classes of anti-hyperglycemic agents, but also between individual agents within a therapeutic classCitation22,Citation25. The SGLT2 inhibitor, canagliflozin, has been shown to improve blood glucose more effectively than a sulfonylureaCitation26 or a DPP-4 inhibitorCitation27–29; canagliflozin reduces body weight compared with a sulfonylureaCitation26 or sitagliptinCitation27; canagliflozin has a lower risk of hypoglycemia than a sulfonylureaCitation26; and canagliflozin 300 mg improves cardiometabolic markers more effectively than other SGLT2 inhibitorsCitation30. Thus, we hypothesized that canagliflozin might be associated with greater persistence and adherence than other anti-hyperglycemic agents. The objective of this study was to compare persistence with and adherence to canagliflozin vs the other agents from all three classes of newer anti-hyperglycemic agents available in the US.
Methods
Study design
This was a retrospective cohort study of administrative claims data from Truven Health Analytics Marketscan Commercial Claims and Encounters and Medicare Supplemental Databases. The Marketscan claims database contains data for more than 77.7 million individuals in the most recent full data year and provides detailed cost, use, and outcomes data for inpatient and outpatient healthcare services and outpatient prescriptions. The commercial database contains data for employees, their spouses, and dependents who are covered by employer-sponsored private health insurance. The Medicare Supplemental database profiles the healthcare experience of retirees with Medicare supplemental insurance paid by employers.
Patient selection criteria
Medical and pharmacy claims data from February 1, 2013 to July 31, 2015 (the study period) were analyzed. Patients were included in the analysis if they had ≥1 claim for a newer anti-hyperglycemic agent between February 1, 2014 and July 31, 2014 (the identification period). As such, the agents considered included: canagliflozin 100 mg, canagliflozin 300 mg, dapagliflozin 5 mg, dapagliflozin 10 mg, sitagliptin, saxagliptin, linagliptin, liraglutide, exenatide, or long-acting exenatide. The index anti-hyperglycemic agent and date for each patient was the patient’s first pharmacy claim in the identification period for one of these agents.
The analysis included adult patients (age ≥18 years on the index date) with continuous enrollment (medical and pharmacy benefits) ≥12 months pre-index and ≥12 months post-index. Eligible patients were assessed until health plan disenrollment, death, or the end of the study period, whichever occurred first. All patients were required to have ≥1 medical claim with a type 2 diabetes mellitus diagnosis (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] code 250.x2) during the study period.
Key exclusion criteria were diagnosis of type 1 diabetes mellitus (ICD-9-CM: 250.x1, 250.x3); >1 anti-hyperglycemic agent on the index date; a claim for any anti-hyperglycemic agent in the same class in the 12-month pre-index period; discharge from inpatient admission to a nursing home; neoplasm (malignant or benign; ICD-9-CM: 140-239); HIV (ICD-9-CM: 042, 079.53, 795.71, V65.44, V08); pregnancy (ICD-9-CM: 633, 646.33, 646.82, 650, 651.03, 651.6, 651.21, 653, 761.4, 779.6, V22.2, V23.87); or a fixed-dose combination of anti-hyperglycemic agents during the post-index period that contained the index anti-hyperglycemic agent.
Assessments
Baseline was defined as the 12-month pre-index period for each patient. Demographic data collected from the databases included age, gender, geographic region (Northeast, North central, South, West, or unknown), and health plan type (comprehensive, exclusive provider organization, health maintenance organization, point of service, preferred provider organization, point of service with capitation, consumer-directed health plan, high-deductible health plan, or unknown). Cost data obtained from the databases were co-payment/co-insurance level for the index anti-hyperglycemic agent and for each anti-hyperglycemic agent during the baseline period, adjusted to 30 days of supply and categorized as $0 to <$15, $15 to <$30, or ≥$30. Clinical characteristics included claims for other anti-hyperglycemic agents during the baseline period (metformin, sulfonylureas, meglitinide derivatives, α-glucosidase inhibitors, thiazolidinediones, DPP-4 inhibitors, GLP-1 agonists, SGLT2 inhibitors, or insulin), diagnosis codes for individual co-morbid conditions (anxiety, chronic ischemic heart disease, depression disorder, diabetic neuropathy, heart failure, hyperlipidemia, hypertension, ischemic stroke, myocardial infarction, nephropathy, peripheral vascular disease, renal diseases, retinopathy, or urinary tract infections), and co-morbidity scores. Four different indices were used to derive the co-morbidity scores during the baseline period: the adapted Charlson Comorbidity Index score based on medical diagnosesCitation31, the Elixhauser Index score based on medical diagnosis-related groupsCitation32, the Chronic Disease score based on pharmacy claimsCitation33, and the adapted Diabetes Complications Severity Index (DCSI) based on medical diagnoses and pharmacy dataCitation34.
Days of supply were obtained from each pharmacy claim for the index anti-hyperglycemic agent. The average days of supply per pharmacy claim was calculated from the total days of supply, divided by the total number of pharmacy claims during the post-index period. The percentage of patients with a 30-, 60-, and 90-day supply of the index anti-hyperglycemic agent on the index date and during the follow-up period were examined for each anti-hyperglycemic agent.
Persistence days were defined as the number of days the patient remained on the index medication (including dose increases) with a gap of no more than 90 days between two pharmacy claims post-index. Each treatment gap between two pharmacy claims was calculated from the end date for one pharmacy claim (determined from the start date and reported days of supply) and the start date for the next pharmacy claim. If the patient did not have a treatment gap of ≥90 days in the 12-month post-index period, then the patient was considered to be persistent on the index anti-hyperglycemic agent throughout follow-up. Sensitivity analyses for persistence rates were conducted with treatment gaps of ≥60 days and ≥30 days. In each of these analyses, a dosing change within the index treatment was not considered as meeting the definition of a treatment gap for non-persistence.
Differences in treatment adherence between index anti-hyperglycemic agents in the 12-month post-index period were assessed with PDC or MPRCitation35. The PDC was defined as the number of days covered by pharmacy claims for the index anti-hyperglycemic agent, divided by 360 days. This approach provided a conservative measure of adherence because it did not double count for overlap due to early refills. Thus, if a patient was covered by more than one pharmacy claim for the index anti-hyperglycemic agent on a given day, that was counted as only 1 day for the PDC calculation, and the days of supply were not summed for all pharmacy claims that were available on that day. The PDC was chosen as the main measure of adherence in this study because it is used as a quality indicator in clinical practiceCitation36. The MPR, which was defined as the sum of the days of supply from all pharmacy claims for the index anti-hyperglycemic agent divided by 360 days, was also used in this study because it is commonly used in adherence researchCitation3. The MPR calculation assumed that if a patient filled a pharmacy claim early then they would not start to take the new prescription until they completed the expected days of supply from the previous pharmacy claim.
Statistical analysis
Statistical analyses were conducted with SAS Version 9.3 (Cary, NC). Study variables were analyzed descriptively and compared by index anti-hyperglycemic agent. P-values were calculated by Chi-square test for dichotomous and polychotomous variables and t-test for continuous variables. Canagliflozin 100 mg was the reference cohort for all pairwise comparisons to the other anti-hyperglycemic agents.
Kaplan-Meier curves used all available post-index data within the study period, including data >12 months post-index, to graphically illustrate treatment persistence in each cohort. Cox proportional hazards model was used for multivariable analysis of a continuous measure of persistence (time to discontinuation). Based on literature review and clinical rationale, independent variables controlled in the Cox proportional hazards model included the demographic, drug cost, and clinical characteristics described above in the Assessments section. The hazard ratio, 95% confidence interval (CI), and p-value were determined for each covariate. The proportional hazard assumption was tested using Kaplan-Meier curves and the Supremum test.
Median and mean values were calculated for treatment adherence (PDC or MPR) in each cohort. Logistic regression was used for multivariable analysis of dichotomous measures of adherence (PDC ≥80% or MPR ≥80% in the 12-month post-index period), with the demographic, drug cost, and clinical characteristics described above in the Assessments section used as control variables. The odds ratio, 95% CI, and p-value were determined for each covariate.
Results
Patient characteristics
A total of 11,961 patients met all patient selection criteria (), including 10,237 (86%) commercially insured patients and 1,724 (14%) patients covered by Medicare Supplemental. Key baseline characteristics are provided by an index anti-hyperglycemic agent cohort in . The proportion of women was 45% for canagliflozin 100 mg (the reference cohort) and ranged from 38–55% in the other cohorts. Mean patient age was 54.3 years for canagliflozin 100 mg and ranged from 52.0–59.5 years in the other cohorts. The mean number of anti-hyperglycemic agents used by each patient in the 12-month baseline period was 3.2 for canagliflozin 100 mg and ranged from 2.4–3.2 in the other cohorts. The mean number of anti-hyperglycemic agent classes used by each patient in the 12-month baseline period was 2.2 for canagliflozin 100 mg and ranged from 1.4–2.2 in the other cohorts. Baseline co-morbidity scores were generally similar across the cohorts; for example, the mean Charlson co-morbidity index score was 2.5 for canagliflozin 100 mg and ranged from 2.4–3.1 in the other cohorts.
Table 1. Key baseline characteristics, index costs, and days of supply by index anti-hyperglycemic agent.
also provides key characteristics for the index anti-hyperglycemic agent, including patient cost at the index date, number of claims in the post-index period for the index anti-hyperglycemic agent, and total days of supply. The mean cost to the patient, which was calculated as co-payment plus co-insurance adjusted to 30 days, ranged from ∼$46–$76 for the index anti-hyperglycemic agent and from ∼$6–$17 for each of the other baseline anti-hyperglycemic agents. In most cohorts, the index claim was either 30 days or 90 days for >90% of patients (including 30-day supplies for 71–89% of patients and 90-day supplies for 11–26% of patients). During the post-index period, 29–44% of patients received at least one 90-day supply of the index anti-hyperglycemic agent. Most of the index claims for long-acting exenatide were 28 or 84 days because of its once-weekly administration schedule. The combined rates for 28-day or 30-day (77%) index claims and 84-day or 90-day (22%) index claims for long-acting exenatide were similar to the corresponding 30-day and 90-day rates for other index anti-hyperglycemic agents. The proportion of patients who filled their index medication only once was lowest for canagliflozin 100 mg (9%) and 300 mg (8%), and ranged from 13–31% for the other anti-hyperglycemic agents. The mean number of pharmacy claims within 12 months post-index was greater for canagliflozin 100 mg (6.8) and 300 mg (6.9) than for the other anti-hyperglycemic agents (range = 3.7–6.2).
Treatment persistence
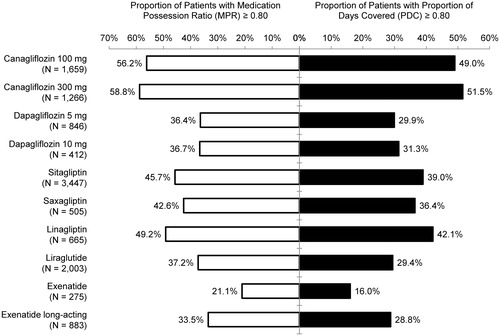
Kaplan-Meier curves for time to non-persistence (i.e. the first treatment gap of ≥90 days between two pharmacy claims post-index) by index anti-hyperglycemic agent are provided in . The proportions of patients persistent on treatment for the first 12 months (p-values compared with canagliflozin 100 mg) were: canagliflozin 100 mg, 61%; canagliflozin 300 mg, 64% (p = 0.037); dapagliflozin 5 mg, 40% (p < 0.001); dapagliflozin 10 mg, 41% (p < 0.001); sitagliptin, 48% (p < 0.001); saxagliptin, 42% (p < 0.001); linagliptin, 52% (p < 0.001); liraglutide, 47% (p < 0.001); exenatide, 23% (p < 0.001); and long-acting exenatide, 39% (p < 0.001).
Figure 2. Kaplan-Meier analysis of persistence, using the time to treatment discontinuation. Cana, canagliflozin; Dapa, dapagliflozin; Exen, exenatide; Exen LA, long-acting exenatide; Lina, linagliptin; Lira, liraglutide; Saxa, saxagliptin; Sita, sitagliptin.
Sensitivity analyses of persistence rates were conducted for 60-day and 30-day treatment gaps during the 12-month post-index period. Using the 60-day treatment gap, 54% of patients persisted on canagliflozin 100 mg, compared with 57% for canagliflozin 300 mg (p > 0.05) and 18–46% for the other cohorts (p < 0.001 for each comparison). Using the 30-day treatment gap, 40% of patients persisted on canagliflozin 100 mg, compared with 43% for canagliflozin 300 mg (p > 0.05) and 11–33% for the other cohorts (p < 0.001 for each comparison).
Cox proportional hazards model results for treatment non-persistence are summarized by index anti-hyperglycemic agent in Supplemental Table S1. Hazard ratios for time to non-persistence favored canagliflozin 100 mg compared with other index anti-hyperglycemic agents (). Patients who initiated treatment with another anti-hyperglycemic agent were 34–159% more likely (each p < 0.05 vs reference) to be non-persistent compared with patients who initiated treatment with canagliflozin 100 mg. The exception was canagliflozin 300 mg, which was associated with a 12% lower hazard of non-persistence than canagliflozin 100 mg (p < 0.05). Other statistically significant (each p < 0.05 vs reference) factors in the model were age (older patients were up to 34% less likely than patients 18–40 years of age to be non-persistent), sex (women were 23% more likely than men to be non-persistent), geographic region (patients in the South were 15% more likely than patients in the Northeast to be non-persistent), and cost of the index anti-hyperglycemic agent (patients who paid $15–<$30 were 9% less likely than patients who paid $0–<$15 to be non-persistent).
Figure 3. Cox proportional hazards model ratios for treatment non-persistence. * p < 0.05 vs reference. † Included combinations with the previous items in the list and excluded combinations with the subsequent items. For example, “DPP-4 inhibitors” included DPP-4 inhibitors either alone or in combination with metformin, sulfonylureas, meglitinides, α-glucosidase inhibitors, and/or thiazolidinediones; and excluded combinations of DPP-4 inhibitors with GLP-1 agonists, SGLT2 inhibitors, or insulin. Co-payment/co-insurance for each pharmacy claim was adjusted to 30 days. CDHP, consumer-directed health plan; CI, confidence interval; DCSI, Diabetes Complications Severity Index; DPP-4 inhibitors, dipeptidyl peptidase-4 inhibitors (e.g. sitagliptin, saxagliptin, linagliptin); EPO, exclusive provider organization; GLP-1 agonists, glucagon-like peptide-1 receptor agonist (e.g. exenatide, liraglutide); HDHP, high-deductible health plan; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; SGLT2 inhibitors, sodium/glucose cotransporter 2 inhibitors (e.g. canagliflozin, dapagliflozin).
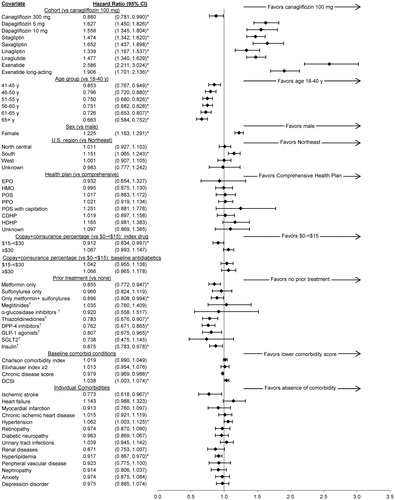
Compared with no anti-hyperglycemic agent in the 12-month baseline period, patients were less likely to be non-persistent on the index anti-hyperglycemic agent if they took metformin plus a sulfonylurea (10% lower), insulin (12% lower), metformin alone (14% lower), a GLP-1 agonist (19% lower), a thiazolidinedione (22% lower), or a DPP-4 inhibitor (24% lower) before the index anti-hyperglycemic agent (; each p < 0.05 vs reference). Higher chronic disease scores were associated with slightly lower hazard of non-persistence (2% lower; p < 0.05). Individual conditions associated with significantly higher or lower hazards of non-persistence (p < 0.05 vs reference) included ischemic stroke (23% lower), hypertension (6% higher), and hyperlipidemia (8% lower). Health plan type, costs of other anti-hyperglycemic agents, other individual co-morbidities, and other co-morbidity scores were not associated with significantly increased or decreased hazards of non-persistence.
Treatment adherence
Median adherence rates for canagliflozin 100 mg (MPR = 0.83; PDC = 0.79) and canagliflozin 300 mg (MPR = 0.92; PDC = 0.81) were greater than for the other index anti-hyperglycemic agents (MPR = 0.33–0.75; PDC = 0.33–0.72) (). Mean values also were greater for canagliflozin than for the other index anti-hyperglycemic agents (; p < 0.001 for the comparison to canagliflozin 100 mg in each cohort except canagliflozin 300 mg).
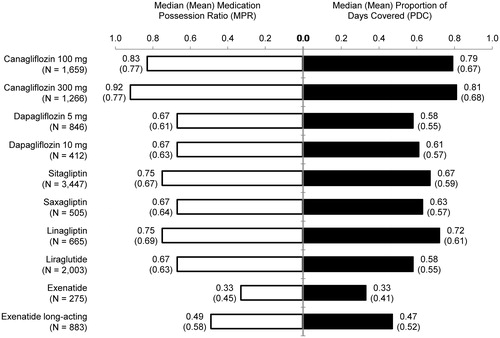
Patients were more likely to be adherent to canagliflozin compared with other index anti-hyperglycemic agents when adherence was measured by either PDC ≥80% or MPR ≥80% within 12 months post-index (). Good treatment adherence defined as PDC ≥80% was observed for 49% and 52% of patients whose index anti-hyperglycemic agent was canagliflozin 100 mg and 300 mg, respectively, and ranged from 16–42% for the other anti-hyperglycemic agents (p < 0.01 for the comparison to canagliflozin 100 mg in each cohort except canagliflozin 300 mg). Good treatment adherence defined as MPR ≥80% was observed for 56% and 59% of patients whose index anti-hyperglycemic agent was canagliflozin 100 mg and 300 mg, respectively, and ranged from 21–49% for the other anti-hyperglycemic agents (p < 0.01 for the comparison to canagliflozin 100 mg in each cohort except canagliflozin 300 mg).
In the multivariable logistic analyses, compared with canagliflozin 100 mg the odds for achieving good adherence were 28–79% lower for PDC (p ≤ 0.001 for each comparison) and 28–78% lower for MPR (p ≤ 0.001 for each comparison) for all other anti-hyperglycemic agents except canagliflozin 300 mg, which was associated with 15% higher odds for achieving good adherence for each measure (p > 0.05) (). Other significant (p < 0.05) predictors of good treatment adherence were older age, male sex, Northeast compared with South, lower cost for the index anti-hyperglycemic agent, lower cost for baseline anti-hyperglycemic agents, higher chronic disease score at baseline, baseline treatment with metformin, a thiazolidinedione, a DPP-4 inhibitor, a GLP-1 agonist, an SGLT2 inhibitor, or insulin, absence of heart failure or hypertension, and presence of ischemic stroke or hyperlipidemia (). Enrollment in a point-of-service health plan predicted poorer treatment adherence compared with comprehensive plans (p < 0.05).
Table 2. Odds ratios for treatment adherence, defined as either proportion of days covered ≥80% or medication possession ratio ≥80%, using multivariable logistic regression analyses.
Discussion
This analysis analyzed treatment persistence and treatment adherence among 11,961 patients in the US with type 2 diabetes mellitus and commercial or Medicare Supplemental insurance who received a new pharmacy claim for canagliflozin or another SGLT2 inhibitor (dapagliflozin), a DPP-4 inhibitor (sitagliptin, saxagliptin, or linagliptin), or a GLP-1 receptor agonist (liraglutide or exenatide) between February 1, 2014, and July 31, 2014. In the base case, treatment persistence was defined as continuous treatment with the index anti-hyperglycemic agent without a ≥90-day treatment gap between pharmacy claims in the 12-month post-index period. Using this definition, the treatment persistence rate was significantly greater for the reference cohort (canagliflozin 100 mg) than for the other anti-hyperglycemic agents. Approximately 61% of patients who received a pharmacy claim for canagliflozin 100 mg in this period were persistent on treatment at 12 months, compared with 23–52% of patients who initiated treatment with another anti-hyperglycemic agent. The only group with greater treatment persistence than canagliflozin 100 mg was the higher dose of canagliflozin 300 mg (64% persistence at 12 months).
A meta-analysis of previous studies of treatment persistence to oral anti-hyperglycemic agents reported rates that ranged from 33–61% in individual studiesCitation3. Results for individual agents in the study reported herein should not be compared directly to results for individual agents in previous studies with different study designs, but the results of this study should be generalizable to other populations because it used a large sample of patients with commercial or Medicare supplemental insurance across the US.
This study also analyzed treatment adherence, which measures the proportion of time that a patient actually takes a drug as intended. Mean treatment adherence rates, as determined from reported days of supply during the 12-month post-index period, were 79–92% for the two doses of canagliflozin and 33–75% for all other anti-hyperglycemic agents in the analysis. These adherence results mirrored those of the meta-analysis of previous studies, which reported a pooled treatment adherence rate of 75% overall for oral anti-hyperglycemic agents, with rates that ranged from 52–88% in individual studiesCitation3. Rates of good adherence in this study, defined as ≥80% treatment adherence during the 12-month post-index period, were ∼50–60% for both canagliflozin cohorts and <50% for all other cohorts. These rates were also consistent with those reported previouslyCitation2,Citation3.
A Cox proportional hazards model for treatment persistence and logistic regressions for treatment adherence were conducted to examine if the index agent continued to have a significant association with treatment patterns after adjustment for other significant predictors of persistence and adherence. After inclusion of the covariates in the models, the index anti-hyperglycemic agent continued to significantly predict treatment patterns, with hazards for treatment non-persistence that were 34–159% greater, and odds for treatment adherence were 28–79% lower for other anti-hyperglycemic agents compared with canagliflozin. Other significant predictors of better treatment persistence and adherence in these models included older age, male gender, geographic region (the Northeast vs South US), higher chronic disease score at baseline, and baseline treatment with selected therapies (metformin, a thiazolidinedione, a DPP-4 inhibitor, a GLP-1 agonist, or insulin). These results were consistent with previous research for predictors of treatment non-adherenceCitation4–6.
The observed influence of cost to the patient (co-payment plus co-insurance) on treatment persistence and adherence in this study was more difficult to interpret. A cost to the patient of ≥$30 (vs $0 to <$15) for the index anti-hyperglycemic agent was not associated with a significantly greater hazard of persistence, but it was significantly related to the odds of adherence (17% lower). In contrast, a cost to the patient of $15 to <$30 (vs $0 to <$15) for the index anti-hyperglycemic agent significantly decreased the hazard of non-persistence by 9% and was not significantly related to the odds of adherence. Previous research has shown that patients with financial difficulties are less likely to adhere to diabetes therapyCitation37. However, this analysis only had access to costs from pharmacy claims and could not analyze each patient’s financial situation overall.
Treatment persistence reflects input from both the prescriber and the patient, both of whom may influence treatment persistence; a prescriber will be more likely to prescribe a drug again if it works well, and a patient will be more likely to keep taking the drug for the same reason. The patient is the primary driver for treatment adherence and, thus, may be influenced by both efficacy and tolerability; a patient who experiences unwanted side-effects from a medication is less likely to take it as prescribed. However, this retrospective analysis of claims data could not examine clinical outcomes such as glucose control and drug tolerability. Thus, additional research is needed to explain the observed differences in persistence and adherence between the index anti-hyperglycemic agents in this study. Previous studies have reported that the reference treatment in this study (canagliflozin) reduces blood glucose more effectively than a sulfonylureaCitation26 or DPP-4 inhibitors (sitagliptinCitation27, saxagliptin, linagliptin, or alogliptin)Citation28,Citation29. Canagliflozin was also shown to reduce body weight and hypoglycemia compared with a sulfonylureaCitation26 and reduced body weight compared with sitagliptinCitation27. Although there are no head-to-head studies of clinical efficacy and safety between SGLT2 inhibitors, results from a randomized, double-blind, cross-over, pharmacodynamic study of healthy participants showed that canagliflozin 300 mg has greater urinary glucose excretion and smaller post-prandial glucose excursions compared with dapagliflozin 10 mg, suggesting improved efficacy for canagliflozin relative to dapagliflozinCitation38. A meta-analysis of 38 studies reported that canagliflozin 300 mg improved cardiometabolic markers more effectively than other SGLT2 inhibitorsCitation30. Collectively, the available evidence suggests that differences in efficacy and tolerability could have contributed to the observed differences in treatment persistence and adherence in this study.
Previous research has shown that patients with better treatment persistence and adherence have better clinical and economic outcomesCitation2,Citation7–13. Thus, significant differences between newer anti-hyperglycemic agents for treatment persistence and adherence may both result from and lead to improved clinical outcomes. As such, a prospective, comparative study would be required to establish a direct association between clinical outcomes and treatment adherence and persistence with anti-hyperglycemic agents.
This study used two commonly used methods to estimate treatment adherence from pharmacy claims. In the first method, PDC was determined from the number of days that a patient was covered by at least one pharmacy claim for the index anti-hyperglycemic agent. Several organizations, including CMS, use adherence to diabetes medications as a quality measure for health plansCitation16–18, and PDC is a commonly used metric to assess treatment adherence in clinical practiceCitation36. Another advantage of PDC is that it provides a conservative estimate for treatment adherence that does not double count for early refills. If a patient is covered by two overlapping pharmacy claims on the same day it is counted as only 1 day of coverage in the PDC. The other measure of treatment adherence in this study, MPR ≥80%, is often used in clinical research. The MPR counts two overlapping pharmacy claims on the same day as 2 days of supply, because it assumes that a patient with an early refill will not start the second pharmacy claim until the days of supply from the first pharmacy claim are done. Thus, the MPR tends to result in higher rates of treatment adherence, which was the case in this study; ∼56–59% of patients who initiated either dose of canagliflozin, and 21–49% of patients who initiated other anti-hyperglycemic agents, achieved MPR ≥80%.
A potential limitation of the base case specification in this study was the use of a long treatment gap (≥90 days) in the definition for treatment persistence. To address this, sensitivity analyses were conducted with shorter treatment gaps of ≥60 days or ≥30 days; these analyses also resulted in significantly better 12-month treatment persistence for canagliflozin compared with other anti-hyperglycemic agents.
Patients with HIV, neoplasms, or pregnancy were excluded from the analysis because these conditions could have a substantial influence on persistence and adherence that was not related to type 2 diabetes or its treatment. Inclusion of those patients could improve the generalizability of the results to all patients with type 2 diabetes. This study also excluded patients who entered a nursing home at any time during the study period, because claims from long-term care pharmacies were not collected in the database. However, patients who were hospitalized during the study were not excluded because pharmacy claim data were available from the database for the hospitalizations. Patients who received another anti-hyperglycemic agent from the same class in the prior 12 months were excluded so the study could examine the new use of an anti-hyperglycemic agent from a given class, but this did not exclude patients who received another anti-hyperglycemic agent from the same class >12 months before the index date. The possible index dates covered only 6 months in early 2014; this period was selected to begin after both SGLT2 inhibitors were available for commercial use and to end when at least 12 months of follow-up data were available for all index dates. Examining anti-hyperglycemic agent use shortly after the SGLT2 inhibitors were both approved may have influenced the study results if prescribers had not prescribed these anti-hyperglycemic agents previously and formularies were not yet updated to include these anti-hyperglycemic agents. Some of the newest anti-hyperglycemic agents were omitted from this analysis, either because too few patients received the agent (alogliptin) or because the agent was approved too recently to be included in the study (albiglutide, empagliflozin, dulaglutide).
Other potential limitations of this study are common to all claims-based studies. Claims data are collected for the purpose of payment and a claim for a filled prescription does not indicate whether the medication was actually consumed or that it was taken as prescribed. Conversely, claims do not reflect the additional use of drugs that are not prescribed and dispensed, such as free samples. Claims may also be subject to bias or coding errors.
Conclusions
This claims-based analysis found that, among patients with type 2 diabetes in the US who initiated treatment with SGLT2 inhibitors, DPP-4 inhibitors, or GLP-1 agonists, canagliflozin was associated with better treatment persistence and treatment adherence than the other newer anti-hyperglycemic agents. Additional research would be required to confirm if better treatment persistence and adherence with canagliflozin are associated with better clinical and economic outcomes.
Transparency
Declaration of funding
Janssen Scientific Affairs, LLC provided funding to STATinMED Research to conduct the analyses.
Declaration of financial/other relationships
JC is an employee of Janssen, a Johnson & Johnson company. WC is also a Janssen employee and owns stock in Johnson & Johnson. OB, YW, and LX are employees of STATinMED Research. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Janssen Scientific Affairs, LLC, provided funding to Jonathan Latham of PharmaScribe, LLC to assist with the preparation and submission of the manuscript.
References
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44-7
- Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One 2014;9:e108145
- Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin 2015;31:1283-96
- Couto JE, Panchal JM, Lal LS, et al. Geographic variation in medication adherence in commercial and Medicare part D populations. J Manag Care Spec Pharm 2014;20:834-42
- Manteuffel M, Williams S, Chen W, et al. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt) 2014;23:112-19
- Tan E, Yang W, Pang B, et al. Geographic variation in antidiabetic agent adherence and glycemic control among patients with type 2 diabetes. J Manag Care Spec Pharm 2015;21:1195-202
- Guillausseau PJ. Influence of oral antidiabetic drugs compliance on metabolic control in type 2 diabetes. A survey in general practice. Diabetes Metab 2003;29:79-81
- Ho PM, Magid DJ, Masoudi FA, et al. Adherence to cardioprotective medications and mortality among patients with diabetes and ischemic heart disease. BMC Cardiovasc Disord 2006;6:48
- Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care 2012;35:1279-84
- McAdam-Marx C, Bellows BK, Unni S, et al. Impact of adherence and weight loss on glycemic control in patients with type 2 diabetes: cohort analyses of integrated medical record, pharmacy claims, and patient-reported data. J Manag Care Spec Pharm 2014;20:691-700
- Ayyagari R, Wei W, Cheng D, et al. Effect of adherence and insulin delivery system on clinical and economic outcomes among patients with type 2 diabetes initiating insulin treatment. Value Health 2015;18:198-205
- Buysman EK, Liu F, Hammer M, et al. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther 2015;32:341-55
- Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521-30
- Institute for Healthcare Improvement (IHI). The IHI Triple Aim. http://www.ihi.org/engage/initiatives/tripleaim/Pages/default.aspx. Accessed February 16, 2016
- Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27:759-69
- Seabury SA, Lakdawalla DN, Dougherty JS, et al. Medication adherence and measures of health plan quality. Am J Manag Care 2015;21:e379-89
- National Quality Forum. Adherence to Oral Diabetes Agents for Individuals with Diabetes Mellitus (NQF#2468). http://www.qualityforum.org/. Accessed January 29, 2016
- Pharmacy Quality Alliance. Executive update on medication quality measures in Medicare part D plan ratings 2013. 2015. http://pqaalliance.org/measures/cms.asp. Accessed January 29, 2016
- American Diabetes Association. 7. Approaches to glycemic treatment. Diabetes Care 2016;39(Suppl 1):S52-S9
- Farr AM, Sheehan JJ, Curkendall SM, et al. Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther 2014;31:1287-305
- Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther 2014;31:1119-33
- Malmenas M, Bouchard JR, Langer J. Retrospective real-world adherence in patients with type 2 diabetes initiating once-daily liraglutide 1.8 mg or twice-daily exenatide 10 mug. Clin Ther 2013;35:795-807
- Pelletier EM, Pawaskar M, Smith PJ, et al. Economic outcomes of exenatide vs liraglutide in type 2 diabetes patients in the United States: results from a retrospective claims database analysis. J Med Econ 2012;15:1039-50
- Yu M, Xie J, Fernandez Lando L, et al. Liraglutide versus exenatide once weekly: Persistence, adherence, and early discontinuation. Clin Ther 2016;38:149-60
- Gibson TB, Song X, Alemayehu B, et al. Cost sharing, adherence, and health outcomes in patients with diabetes. Am J Manag Care 2010;16:589-600
- Leiter LA, Yoon KH, Arias P, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care 2015;38:355-64
- Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013;36:2508-15
- Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582-92
- Thayer S, Chow W, Korrer S, et al. Real-world evaluation of glycemic control among patients with type 2 diabetes mellitus treated with canagliflozin versus dipeptidyl peptidase-4 inhibitors. Curr Med Res Opin 2016;32:1087–96
- Zaccardi F, Webb DR, Htike ZZ, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes Metab 2016. doi: 10.1111/dom.12670
- Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008;61:1234-40
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27
- Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol 1992;45:197-203
- Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15-23
- Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013;51:S11-S21
- Pharmacy Quality Alliance. PQA medication quality measures in the health insurance marketplace: Quality rating system (QRS) beta test measure set. http://pqaalliance.org/measures/qrs.asp. Accessed January 27, 2016
- Tiv M, Viel JF, Mauny F, et al. Medication adherence in type 2 diabetes: the ENTRED study 2007, a French Population-Based Study. PLoS One 2012;7:e32412
- Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab 2015;17:188-97