Abstract
Objectives: To estimate economic impact resulting from increased biologics use for treatment of rheumatoid arthritis (RA) and Crohn’s disease (CD) in Argentina, Brazil, Colombia, and Mexico.
Methods: The influence of increasing biologics use for treatment of RA during 2012–2022 and for treatment of CD during 2013–2023 was modeled from a societal perspective. The economic model incorporated current and projected medical, indirect, and drug costs and epidemiologic and economic factors. Costs associated with expanded biologics use for RA were compared with non-expanded use in Argentina, Brazil, Colombia, and Mexico. A similar analysis was conducted for CD in Brazil, Colombia, and Mexico.
Results: Accounting for additional costs of biologics and medical and indirect cost offsets, the model predicts that expanded use of biologics for patients with RA from 2012 to 2022 will result in cumulative net cost savings of ARS$2.351 billion in Argentina, R$9.004 billion in Brazil, COP$728.577 billion in Colombia, and MXN$18.02 billion in Mexico; expanded use of biologics for patients with CD from 2013 to 2023 will result in cumulative net cost savings for patients with CD of R$0.082 billion in Brazil, COP$502.74 billion in Colombia, and MXN$1.80 billion in Mexico. Indirect cost offsets associated with expanded biologics use were a key driver in reducing annual per-patient net costs for RA and CD.
Limitations: Future economic projections are limited by the potential variance between projected and actual future values of biologic prices, wages, medical costs, and gross national product for each country.
Conclusions: Increasing biologics use to treat RA and CD may limit cost growth over time by reducing medical and indirect costs. These findings may inform policy decisions regarding biologics use in Argentina, Brazil, Colombia, and Mexico.
Introduction
Latin America, like other developing regions, has experienced a shift in the allocation of healthcare resources from acute to chronic diseases, as chronic diseases have become the major contributor to morbidity and mortalityCitation1,Citation2. In particular, chronic inflammatory diseases such as rheumatoid arthritis (RA), a systemic inflammatory disease affecting the synovium of joints, tendons, and some extra-articular sitesCitation3, and Crohn’s disease (CD), characterized by focal, asymmetric, transmural inflammation of the gastrointestinal tractCitation4, are particularly debilitating and pose a large societal economic burden as populations grow and life expectancy increases.
The economic burden of RA and CD is associated primarily with indirect and direct medical costsCitation5. Given the disability associated with RA and a typical age of onset during working years, indirect costs of RA may exceed direct costs in some countriesCitation5. Indirect costs of RA were estimated to be $10.9 billion in 2005 USD$Citation6, with individuals with RA being 53% less likely to be employed and spending 3.6-times more days ill in bed compared with individuals without RACitation7. Direct medical costs associated with RA are also substantial and vary by countryCitation5. The health burden of RA may vary by socioeconomic factors, with low per-capita income countries having a higher burden of diseaseCitation5. Patients with CD experience flares followed by periods of remission. Flares may require urgent medical attention (e.g. hospitalization and surgery) that contributes to the direct costs of the disease, but periods of remission are not necessarily symptom free and unpredictable episodes of pain and abdominal cramping significantly impair quality-of-lifeCitation8. As with RA, many patients with CD are of workforce age, and the symptom burden of CD may lead some patients to work only part-time or leave the workforce entirelyCitation8, which contributes to the substantial economic burden of the disease.
The prevalence of RA in Latin America ranges from 0.4–1.6%Citation3, and, although prevalence over time is expected to remain constant, a trend toward an earlier age of onset of RA in Latin American countries may have societal cost implicationsCitation9. The economic burden of RA will also increase as the world’s population agesCitation5. The published prevalence of CD is 5.65 cases per 100,000 persons in Brazil; however, reliable prevalence data in other Latin American countries are scarceCitation10, and the current prevalence of CD is likely much higher in Brazil and other Latin American countries. Worldwide, an increasing prevalence of CD seems to be associated with changes in environment and a “Westernized” lifestyleCitation4,Citation11; for example, CD is more common in Hispanics living in the US than in Latin AmericaCitation12.
Both non-biologic and biologic disease-modifying anti-rheumatic drugs (DMARDs) have been demonstrated to reduce signs and symptoms of RA, reduce work disability, and prevent joint damageCitation13,Citation14. New RA treatment guidelines for Argentina, Brazil, Colombia, and Mexico could contribute to increased utilization of biologics in these countriesCitation15–18. For example, the first early arthritis cohort in Argentina, the Argentine Consortium for Early Arthritis (CONAART), has demonstrated that disease activity is the main disease variable associated with work disability in patients with RACitation19, and treat-to-target guidelines have been developed to advocate achievement of remission or low disease activity as the treatment goal for patients with RACitation20. For patients with moderate-to-severe CD, use of immunomodulators and biologics is recommended to first treat acute disease, then induce clinical remission and ultimately maintain response and remissionCitation4,Citation21. Of note, use of biologics, such as tumor necrosis factor (TNF) antagonists, has been associated with improved clinical outcomes and reduced medical and indirect costs for patients with moderate-to-severe RA and CD22–26.
The economic environment in developing regions is rapidly changing. In Latin America, gross domestic product (GDP) and total healthcare spending are increasing in Argentina, Brazil, Colombia, and MexicoCitation27–30. As a percentage of GDP, real average wages have increased more than 50% since 2002 in Argentina, Brazil, Colombia, and MexicoCitation31–34. Rising medical costs and rising wages will likely result in an increased economic burden of RA and CD in these countriesCitation29,Citation30,Citation33,Citation35–37.
Although common in Europe, the US, and Canada, access to care and early intervention to treat inflammatory disorders such as RA and CD can be difficult to achieve in Latin America, which has a fragmented healthcare systemCitation3. As populations expand and life expectancy increases, Latin American countries with state-run healthcare programs increasingly face decisions about allocation of scarce resources for the optimal management of debilitating chronic inflammatory diseases. Our objective was to project the economic impact from a societal perspective of expanding the use of biologics for the treatment of RA and CD in Argentina, Brazil, Colombia, and Mexico over a 10-year period.
Methods
An economic model was designed to demonstrate the potential cost offsets of expanding biologics use for the treatment of patients with RA and CD in Argentina, Brazil, Colombia, and Mexico. These countries were selected because they are the four countries with the largest populations in the Latin American regionCitation38. Seven individual models were developed using the same Excel-based model framework to project the impact of expanded biologics use among patients with RA in Argentina, Brazil, Colombia, and Mexico and among patients with CD in Brazil, Colombia, and Mexico. The impact of expanded biologics use among patients with CD was not modeled for Argentina because of limitations associated with country-specific economic data that were discovered after developing the RA modelCitation39. Model inputs were country- and disease-specific based on available data; if country- and/or disease-specific data were not available, we assumed the closest possible scenario. The RA model included the following biologics that were approved for the treatment of moderate-to-severe RA in Argentina, Brazil, Colombia, and Mexico as of 2012: abatacept, adalimumab, certolizumab pegol, etanercept (including a biosimilar version), golimumab, infliximab, rituximab (including a biosimilar version in Mexico), and tocilizumab. The CD model included the following biologics that were approved for the treatment of moderate-to-severe CD as of 2013: adalimumab, certolizumab pegol, and infliximab in Brazil and Mexico and adalimumab and infliximab in Colombia.
The target population for the model included all patients with RA in Argentina, Brazil, Colombia, and Mexico and all patients with CD in Brazil, Colombia, and Mexico. We used a societal perspective to develop the economic model, which compared costs in two scenarios for both diseases over a 10-year time horizon (2012–2022 for RA and 2013–2023 for CD; model development began in 2012 for RA and 2013 for CD). First, we evaluated an expanded biologic use scenario, in which the biologic penetration rate was greater in 2022 for patients with RA and 2023 for patients with CD than corresponding biologic penetration rates in 2012 (for RA) and 2013 (for CD). Second, we evaluated a non-expanded biologic use scenario, in which the biologic penetration rates in 2022 and 2023 were the same as in 2012 and 2013 for RA and CD, respectively.
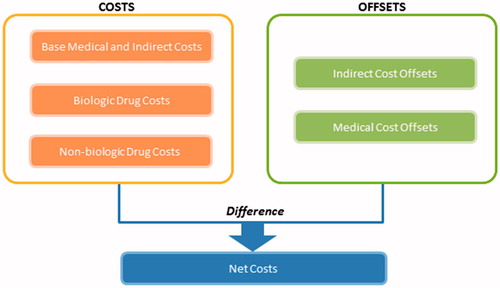
The cost elements of the model were selected to reflect potential costs as well as cost offsets associated with the treatment of RA and CD. For all patients, costs included direct (non-drug) medical costs, indirect costs, biologic drug costs, and non-biologic drug costs. Potential cost offsets included reductions in direct medical and indirect costs achieved through effective biologic therapy in patients with RA and CD. The primary model inputs were the base annual medical (non-drug) and indirect costs per patient. Average annual per-patient costs of RA or CD therapy as well as cost offsets were incorporated to estimate a total cost per patient. Drug costs were calculated using prices for 1 year of biologic and non-biologic therapy (based on dosing in product labeling and weighted based on product market share) and an average medication adherence rate (dosing information for single drugs that were used to calculate drug costs for RA and CD are provided in Supplementary Tables 1 and 2, respectively). Biologic adherence rates were calculated using a blended adherence rate based on drug-specific adherence rates for those that were included in the model, where adherence was weighted based on the number of patients in the country using drugs. Annual per-patient costs were aggregated to estimate total annual costs among RA and CD patients in each country using country-specific population size and disease prevalence.
Table 1. Components of an economic model designed from a societal perspective to compare costs associated with expanded vs non-expanded biologics use in patients with rheumatoid arthritis in Brazil.
Table 2. Components of an economic model designed from a societal perspective to compare costs associated with expanded vs non-expanded biologics use in patients with Crohn’s disease in Brazil.
Additional elements of the model included epidemiologic, economic, and cost factors. In both the expanded and non-expanded biologic use scenarios, the model incorporated projected changes in population size, GDP, personal income, consumer price index (CPI), disease prevalence, medical costs, indirect costs, and drug costs. The model also incorporated projected changes in treat-to-target (T2T) use in the case of RA. Additional benefits (medical and indirect cost reductions) attributed to biologic use were included in both expanded and non-expanded biologic use scenarios.
The specific elements for each input of the model are summarized in for RA and for CD, using Brazil as an example. In addition, illustrates the net cost calculation for each year. A full description of these elements for the other countries is provided in and . The economic burden of each disease was accounted for by disease prevalence and projected growth in prevalence rates, drug costs and projected drug cost changes, and direct and indirect costs and their associated growth rates. Country-specific macroeconomic factors included the total population of each country and the population growth rate; GDP and projected GDP growth rate and CPI growth rates; and unemployment and projected growth in personal income (indirect costs). Treatment use factors included in the model were biologic use and growth of biologic use as well as a treatment adherence rate. The cost offsets attributed to biologic therapy were considered in the model. Patent expiration and its potential impact on prices were taken into account in the price change in biologics over time, where the prices of biologics are assumed to decline over time.
Table 3. Components of an economic model designed from a societal perspective to compare costs associated with expanded vs non-expanded biologics use in patients with rheumatoid arthritis in Argentina, Colombia, and Mexico.
Table 4. Components of an economic model designed from a societal perspective to compare costs associated with expanded vs non-expanded biologics use in patients with Crohn’s disease in Colombia and Mexico.
Outputs of the economic model were as follows: annual total cost per patient (including medical [non-drug] costs, indirect costs, and drug costs for RA and CD in each country) and annual total cost for all RA and CD patients in each country as a percentage of GDP. Different sensitivity analyses were run in different models to evaluate the effect of model variables, such as disease prevalence rate, growth in disease prevalence rate, T2T rate (for RA), biologic penetration rate, and medication adherence rate. For example, in Colombia, medication adherence rates were evaluated, but not T2T rates.
In the analysis assessing costs for RA patients in Brazil, the R$51,329 value in represents 2012 biologic drug costs; whereas, R$1,979 in Supplemental Table 3 is the discounted value (to 2012) of biologic drug costs in 2022. To relate these two numbers, it is important to consider the 5.39% assumed biologic price decline over time and the increase in biologic use over time. In more detail, the deflated biologic drug cost in 2022 (R$1,979) is the product of medication adherence, average annual cost of biologic drugs per RA patient, and biologic use among all RA patients. In this example, the medication adherence rate was assumed to be 78% for RA patients, and the average annual cost of biologic drugs per RA patient was calculated to be R$51,329 in 2012 and decreasing at a rate of 5.39% per year. As a result, the average annual cost of biologics per RA patient in 2022 was calculated to be R$29,481, which was in turn used to calculate the deflated value (R$1,979). Similarly, the biologic use rate among all RA patients was calculated to be 4.5% per year, resulting in an average annual cost of biologics per RA patient of R$29,481 in 2022, which was then used to calculate the deflated value (R$1,979).
Cost data are reported in Brazilian reals (R$), Argentine pesos (ARS$), Colombian pesos (COP$), and Mexican pesos (MXN$). For RA, cost data are reported in 2012 calendar-year values, and, for CD, cost data are reported in 2013 calendar-year values. When available, application of country-specific discount rates recommended for health technology assessments were used to evaluate the overall return on investment (ROI) for expanded biologics use in each country.
Results
Total annual societal costs for RA and CD in Argentina, Brazil, Colombia, and Mexico are significant. As a percentage of GDP, the current annual costs range from 0.33–0.71% for RA (2012 costs) in Argentina, Brazil, Colombia, and Mexico and from 0.01–0.09% for CD (2013 costs) in Brazil, Colombia, and Mexico. For patients with RA, biologic drug costs account for 9% of total annual costs in Argentina, 11% in Brazil, 7% in Colombia, and 10% in Mexico. Based on the model, biologic drug costs account for 29% of the total annual costs in patients with CD in Brazil, 2.7% in Colombia, and 11% in Mexico.
Our model demonstrated cumulative net cost savings among patients with RA and CD. For patients with RA, the model predicts that expanded use of biologics from 2012 to 2022 will result in a cumulative net cost savings of ARS$2.351 billion in Argentina () (0.518 billion 2012 USD), R$9.004 billion in Brazil () (4.617 billion 2012 USD), COP$728.577 billion in Colombia () (0.405 billion 2012 USD), and MXN$18.02 billion in Mexico () (1.368 billion 2012 USD), accounting for additional cost of biologics and medical cost, indirect cost, and T2T offsets. For CD, expanded use of biologics from 2013 to 2023 will result in a cumulative net cost savings of R$0.082 billion in Brazil () (0.038 billion 2013 USD), COP$502.74 billion in Colombia () (0.269 billion 2013 USD), and MXN$1.80 billion in Mexico () (0.141 billion 2013 USD), accounting for additional cost of biologics and medical and indirect cost offsets. Expressed as ROI, for every additional dollar spent on biologics, the model projects more than 1 dollar of savings through cost offsets (). Using country-specific discount rates (Brazilian discount rates were used for Argentina) ranging from 3% to 10%, expanded biologic use maintains an ROI of greater than 1 for both disease states ().
Figure 2. Cumulative net cost savings from 2012–2022 for patients with rheumatoid arthritis. (a) Argentina. (b) Brazil. (c) Colombia. (d) Mexico. All figures present results of a model and are subject to the assumptions and limitations of that model. [1] No annual discount rate was used for results presented in each figure. [2] Indirect costs include work absenteeism, permanent work disability, and housework lost for housewives, using the human capital approach. Medical costs include costs of hospitalization, drugs, medical and allied health professional visits, and outpatient procedures. [3] Indirect costs include the workloss costs of absenteeism, sick leave, and early retirement due to rheumatoid arthritis, estimated using the human capital approach. Medical costs include costs of outpatient appointments, hospitalizations, diagnostic tests, intra-articular injection sessions, devices and aids, and transportation. [4] Indirect costs were estimated in relation to direct costs using a ratio of indirect-to-direct costs of 2.7:1. Medical costs include orthopedic and other surgeries, laboratory tests, hospitalizations, medical consultations, diagnostic imaging and other tests, physiotherapy, and other costs. [5] Indirect costs include the costs due to job loss. Medical costs include costs of alternative therapies, laboratory/radiological tests, hospitalizations, aid devices, third party help, and transportation.
![Figure 2. Cumulative net cost savings from 2012–2022 for patients with rheumatoid arthritis. (a) Argentina. (b) Brazil. (c) Colombia. (d) Mexico. All figures present results of a model and are subject to the assumptions and limitations of that model. [1] No annual discount rate was used for results presented in each figure. [2] Indirect costs include work absenteeism, permanent work disability, and housework lost for housewives, using the human capital approach. Medical costs include costs of hospitalization, drugs, medical and allied health professional visits, and outpatient procedures. [3] Indirect costs include the workloss costs of absenteeism, sick leave, and early retirement due to rheumatoid arthritis, estimated using the human capital approach. Medical costs include costs of outpatient appointments, hospitalizations, diagnostic tests, intra-articular injection sessions, devices and aids, and transportation. [4] Indirect costs were estimated in relation to direct costs using a ratio of indirect-to-direct costs of 2.7:1. Medical costs include orthopedic and other surgeries, laboratory tests, hospitalizations, medical consultations, diagnostic imaging and other tests, physiotherapy, and other costs. [5] Indirect costs include the costs due to job loss. Medical costs include costs of alternative therapies, laboratory/radiological tests, hospitalizations, aid devices, third party help, and transportation.](/cms/asset/43e42aae-8214-4ae7-aa8f-cc6328e38bb2/ijme_a_1209508_f0002_c.jpg)
Figure 3. Cumulative net cost savings from 2013–2023 for patients with Crohn’s disease. (a) Brazil. (b) Colombia. (c) Mexico. [1] No annual discount rate was used for results presented in each figure. [2] All figures present results of a model and are subject to the assumptions and limitations of that model. [3] Indirect costs include the workloss costs of absenteeism, presenteeism, and unemployment due to Crohn’s disease, estimated using the human capital approach. Medical costs include costs of outpatient appointments, hospitalizations, and diagnostic tests.
![Figure 3. Cumulative net cost savings from 2013–2023 for patients with Crohn’s disease. (a) Brazil. (b) Colombia. (c) Mexico. [1] No annual discount rate was used for results presented in each figure. [2] All figures present results of a model and are subject to the assumptions and limitations of that model. [3] Indirect costs include the workloss costs of absenteeism, presenteeism, and unemployment due to Crohn’s disease, estimated using the human capital approach. Medical costs include costs of outpatient appointments, hospitalizations, and diagnostic tests.](/cms/asset/cd34356b-1534-4a15-b364-0083e1d84d17/ijme_a_1209508_f0003_c.jpg)
Table 5. Return on investment from expanded biologic use over a 10-year period in patients with rheumatoid arthritis or Crohn’s disease in Argentina, Brazil, Colombia, and Mexico.
We also analyzed annual total costs of RA and CD patients as a percentage of GDP. For patients with RA, the model suggests that expanded use of biologics will reduce the percentage of GDP spent on patients with RA from 0.3934% to 0.2749% (vs 0.2877% for non-expanded use in Argentina), from 0.3306% to 0.2639% (vs 0.3050%) in Brazil, and from 0.7117% to 0.6233% (vs 0.6439% for non-expanded use) in Mexico. In Colombia, the model indicates that expanded use of biologics can slow the rate of increase in the percentage of GDP spent on patients with RA from 0.4875% to 0.5019% (vs 0.5470%). The impact of expanded biologic use on the percentage of GDP spent on patients with CD was small in Brazil, Colombia, and Mexico.
The model predicts slower growth in annual costs per RA patient in Argentina (Supplementary Figure 1A), Brazil (Supplementary Figure 1B), Colombia (Supplementary Figure 1C), and Mexico (Supplementary Figure 1D) with expanded use of biologics; the cost savings are driven by medical cost, indirect cost, and T2T offsets. Similarly, growth in annual costs per CD patient in Brazil (Supplementary Figure 2A), Colombia (Supplementary Figure 2B), and Mexico (Supplementary Figure 2C) will be lower with expanded use of biologics, with cost savings driven by medical and indirect cost offsets.
The increased annual cost offsets per patient are summarized in Supplemental Table 3. From 2012 to 2022, the reduction in total costs per RA patient associated with expanded biologics use is mainly due to medical and indirect cost offsets. Similarly, from 2013 to 2023, the reduction in total costs per CD patient with expanded use of biologics is due to the offsets in medical and indirect costs.
Various sensitivity analyses (e.g. increased or decreased disease prevalence, increased growth in RA or CD prevalence over the 10-year horizon, higher biologic penetration rates, lower medication adherence rates, higher treat-to-target rates for patients with RA) did not alter the main conclusions. For example, using a biologic penetration rate of 20.0% in 2022 for patients with RA in Brazil instead of the base-case rate of 13.56% resulted in a reduction in projected annual costs per RA patient from BRL $18,760 (base case) to BRL $16,684 for the expanded biologic use scenario and no change in projected annual costs from the base-case scenario for the non-expanded use scenario. Results of this particular sensitivity analysis followed a similar pattern for Argentina, Colombia, and Mexico, although the reduction in projected annual costs per RA patient in Argentina using a 20.0% biologic penetration rate was of a larger magnitude than the relative reduction in costs in Brazil.
Discussion
RA imposes a significant economic burden in Argentina, Brazil, Colombia, and Mexico. For RA, annual 2012 medical costs per patient were estimated to be ARS$12,214 in ArgentinaCitation37,Citation40, R$6,023 in BrazilCitation40–42, COP$2,870,643 in ColombiaCitation43,Citation44, and MXN$30,673 in MexicoCitation45,Citation46. Annual indirect costs for patients with RA are estimated to be ARS$18,414 in ArgentinaCitation31,Citation40, R$8,931 in BrazilCitation33,Citation47,Citation48, COP$7,750,735 in ColombiaCitation43,Citation44,Citation49 (based on a 2.7:1 ratio of indirect to direct costs), and MXN$25,783 in MexicoCitation45,Citation46.
Although the prevalence of CD is less than that of RA, CD also imposes a significant economic burden in Argentina, Brazil, Colombia, and Mexico. For CD, annual 2013 medical costs per patient are estimated to be R$5,669 in Brazil, COP$4,624,821 in Colombia, and MXN$31,443 per patient in MexicoCitation50,Citation51. Annual indirect costs for patients with CD are R$11,237 per patient in Brazil, COP$7,482,899 per patient in Colombia, and MXN$35,853 per patient in MexicoCitation24,Citation26,Citation51–53.
Our economic model demonstrates that expanding biologic use among patients with RA and CD in Argentina, Brazil, Colombia, and Mexico may lead to a slower growth in total costs of RA and CD over a 10-year horizon as compared with not expanding biologic use. Of course, expanding biologic use will increase drug costs, and medical and indirect costs will increase as the economy of each country grows. However, the treatment benefits associated with expanded biologic use will offset the increase in medical and indirect costs that result from economic growth. Overall, the net reduction in medical and indirect costs will offset the drug costs. The key driving factor in annual net cost savings per patient with RA were the indirect cost offsets associated with expanded biologics use in Argentina, Brazil, and Colombia; in Mexico, medical cost offsets accounted for a greater percentage of the net cost savings. The model predicted that the greatest reduction in annual indirect cost offsets for expanded biologics use among patients with RA will be in Colombia (COP$2,042,126 to COP$618,153). For patients with CD, the magnitude of the difference in net annual cost savings per patient with expanded vs non-expanded biologics use was higher in Colombia and Mexico and lower in Brazil compared with model projections for patients with RA. As with RA, the key factor driving that projected annual net cost savings of expanded biologics use in patients with CD was indirect cost offsets, at least in Brazil and Colombia; in Mexico, the indirect and medical cost offsets were similar. According to the model, the greatest net cost savings percentage-wise with expanded biologics use over a 10-year period will be achieved for patients with CD in Colombia, where annual net costs per patient in 2023 will decrease from COP$18.658 M to COP$15.701 M, and the least annual net cost savings percentage-wise will be achieved for patients with CD in Mexico, where the model projects that annual net costs per patient in 2023 will decrease from MXN$82,170 to MXN$76,353.
This economic model-based evaluation has several strengths. To the authors’ knowledge, this is the first published report of an economic evaluation model from the societal perspective that quantifies the economic value of biologic treatment for the serious autoimmune conditions RA and CD in Argentina, Brazil, Colombia, and Mexico over a 10-year period. The model was comprehensive in that it included epidemiologic and economic disease-related inputs, macroeconomic inputs, treatment-use inputs (i.e. medication adherence, treat-to-target strategy for RA), and treatment effects (i.e. cost offsets from biologic therapy). Also, to the best of our ability, we used local country-specific data for all elements of the model, and country-specific discount rates recommended for health technology assessments to calculate a 10-year ROI associated with expanded use of biologics to treat RA and CD in all countries analyzed. The societal perspective of our model provides the broadest view of the potential economic impact of expanding biologic use among patients with disabling inflammatory disorders, and the conclusions of the study did not change with several sensitivity analyses.
The model included estimations of indirect costs, which are particularly important for developing nations with increasing wages and GDPs. Numerous reports have shown that treatment with biologics in both RA and CD patients has also been shown to reduce indirect costsCitation22,Citation26,Citation54–56. Given that the indirect costs of RA and CD are higher than direct medical costs in many countries, the economic burden associated with reduced work productivity is substantial, and our model confirmed that the cost offsets associated with reduced indirect costs are substantial over a 10-year horizon.
In addition, our model incorporated potential cost offsets from the treat-to-target disease management strategy that has been recommended for patients with RA. The treat-to-target goal is to achieve low disease activity and/or remission, and progress toward this goal may result in more patients receiving biologic medications. We felt that inclusion of the treat-to-target strategy for patients with RA was the best method to model dose optimization of biologics.
Despite the strengths of this model, which projected costs associated with expanding biologics use in patients with RA and CD over a 10-year period, all economic models have various limitations and weaknesses. The model used data and assumptions obtained from the published medical literature; data on file at AbbVie; and data published by various institutions, including the World Bank, the World Health Organization, and the International Monetary Fund. The results, therefore, are subject to the limitations of these data sources and must be viewed in the context of the underlying model assumptions. For Argentina, this model uses officially reported GDP and CPI data. However, there is uncertainty regarding the economic data reported by Argentina. Uncertain inflation rates make precise economic projections difficult, and the conclusions of this model may be impacted by major changes in inflation rate. The model is intended to examine project costs within a range of input values, but may not be applicable, for example, if biologic uptake were to expand greatly beyond assumed uptake. Results of the economic model are also subject to the inherent limitations of future economic projections, such as the potential variance between projected and actual future values of biologic prices, wages, medical costs, and GDP for each country.
Another limitation is that use of data from different published studies of patients with RA and CD assumes that the populations across studies were comparable, and that patients in each country in the model are similar to the populations in the studies. However, for a geographically large country such as Brazil, inflammatory disease prevalence varies between the northern and southern regions of the country. In addition, reliable CD prevalence data for Latin American countries are scarce, and prevalence estimates used in the model may under-estimate actual current and future CD prevalence. Even for countries where disease prevalence rates are reported, such as Brazil, the prevalence in northern and southern portions of the country varies greatly, so the local impact of an economic model may differ somewhat from the results presented here.
It should be noted that Argentina, Brazil, Colombia, and Mexico have different healthcare systems with different proportions of the population being publicly insured, privately insured, or uninsured. For example, in Brazil, the majority of the population is covered by an insurance or health plan, whereas, in Mexico and Argentina, a larger proportion of patients are uninsured or have restricted coverage within their health insuranceCitation79. This can have an impact on access to care, access to specialist care, and access to biologic treatments. To be as comprehensive as possible in our economic analysis, we used model inputs that were country- and disease-specific based on currently available data, and we evaluated expanded vs non-expanded biologic use scenarios in which the biologic penetration rate was varied.
For the RA model, assumptions were made for current and projected treat-to-target prevalence rates and associated cost offsets because these data were not available for Argentina, Brazil, Colombia, and Mexico. However, sensitivity analyses for Argentina, Brazil, and Mexico showed that the results were not sensitive to these inputs. While the treat-to-target strategy was used to model dose optimization of biologics for the treatment of RA, there is no established parallel strategy to model dose optimization of biologics for the treatment of CD. When applying the model to patients with CD in Colombia and Mexico, assumptions were made for current and projected biologic use and associated offsets because these data were not available. In addition, changes in diagnostic rates and treatment practices may affect the future economic and epidemiologic landscape of CD and RA in all Latin American countries.
Our model used a societal perspective to project future costs of expanded vs non-expanded use of biologics in developing regions. Use of a societal perspective is particularly important given the growing populations of developing nations. Also, chronic diseases increasingly affect populations of Latin American countries as life expectancy increases and acute diseases (infections) become less problematic. The expanding GDP and rising wages of Latin American countries are associated with increasingly significant productivity impacts associated with chronic debilitating immune disorders (absenteeism, presenteeism, etc.). Governments of developing nations will increasingly be forced to balance scarce resources with rising healthcare costs.
The 10-year horizon of our model can provide useful ROI projections for government agencies or other payers who are managing limited healthcare resources, and multiple future applications of the model can be envisioned. Biologic medications used to treat RA and CD are indicated for the treatment of several other rheumatologic, dermatologic, and gastrointestinal inflammatory disorders. Our economic model could be adapted as new biologics become available in Argentina, Brazil, Colombia, and Mexico or other developing regions and to predict the impact of expanding biologic use for other inflammatory disorders in Latin America. In addition, the model inputs could be adapted for countries in other developing nations, with availability of reliable public data for various model inputs being the limiting factor.
Conclusions
Increasing biologics use to treat RA and CD may limit cost growth over time by reducing medical and indirect costs. These findings may inform policy decisions regarding biologics use in Argentina, Brazil, Colombia, and Mexico, both for RA and CD and for other serious autoimmune conditions for which biologics are a treatment option.
Transparency
Declaration of funding
This study and all data analyses were funded by AbbVie, Inc. AbbVie authors participated in the study design, the analysis plan, interpretation of the data, preparation review, and approval of the submitted manuscript.
Declaration of financial/other relationships
PGK is a speaker for AbbVie, Janssen-Cilag, Takeda, and Ferring. JRS is a speaker for Abbvie and Pfizer, and declares honoraria received for participating at symposia and advisory boards organized by AbbVie. NT, MS, LC, and YB are employees and stockholders of AbbVie. JC was an employee and stockholder of AbbVie at the time this research was conducted. DM and JS are employees of Analysis Group, which received payment from AbbVie to conduct this research. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Dana L. Randall, MS, PharmD, of Intuitive Graphite Inc., and Eric Bertelsen, PhD, of Arbor Communications, Inc., Ann Arbor, MI, for assistance with manuscript preparation. AbbVie, Inc., North Chicago, IL, provided financial assistance for medical writing and editorial support.
References
- Mody GM, Cardiel MH. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Res Clin Rheumatol 2008;22:621-41
- Bloomberg School of Public Health, Johns Hopkins University. Noncommunicable chronic diseases in Latin America and the Caribbean. Buenos Aires, Argentina: The Healthy Caribbean Coalition Website; 2009. http://www.healthycaribbean.org/publications/documents/NCD-in-LAC-USAID.pdf. Accessed April 10, 2015
- Burgos-Vargas R, Catoggio LJ, Galarza-Maldonado C, et al. Current therapies in rheumatoid arthritis: a Latin American perspective. Reumatol Clin 2013;9:106-12
- Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol 2009;104:465-83
- Gulácsi L, Brodszky V, Baji P, et al. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol 2015;11(1 Suppl):43-52
- Birnbaum H, Pike C, Kaufman R, et al. Societal cost of rheumatoid arthritis patient in the US. Curr Med Res Opin 2010;26:77-90
- Sullivan PW, Ghushchyan V, Huang XY, et al. Influence of rheumatoid arthritis on employment, function, and productivity in a nationally representative sample in the United States. J Rheumatol 2010;37:544-9
- Gater A, Kitchen H, Heron L, et al. Development of a conceptual model evaluating the humanistic and economic burden of Crohn's disease: implications for patient-reported outcomes measurement and economic evaluation. Expert Rev Pharmacoecon Outcomes Res 2015;15:643-56
- Ramos-Remus C, Sierra-Jimenez G, Skeith K, et al. Latitude gradient influences the age of onset of rheumatoid arthritis patients. Clin Rheumatol 2007;26:1725-8
- Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol 2009;46:20-5
- Appleyard CB, Hernández G, Rios-Bedoya CF. Basic epidemiology of inflammatory bowel disease in Puerto Rico. Inflamm Bowel Dis 2004;10:106-11
- Lattimer LD, Chandler MB, Borum ML. Hispanics and inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1214-18
- Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625-39
- Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492-509
- da Mota LM, Cruz BA, Brenol CV, et al. 2012 Brazilian Society of Rheumatology Consensus for the treatment of rheumatoid arthritis. Rev Bras Reumatol 2012;52:152-74
- Grupo de Estudio de Artritis Reumatoidea. Actualización de las guías de práctica clínica en el tratamiento de la artritis reumatoidea. Buenos Aires, Argentina: Sociedad Argentina de Reumatología; 2013. http://www.reumatologia.org.ar/docs/guias_sar_2013.pdf. Accessed April 10, 2015
- Mora KC, González A, Quintana LG. Guide for treatment of early rheumatoid arthritis in a university hospital in Colombia. Rev Colomb Reumatol 2008;15:79-91
- Gobierno Federal de Estados Unidos Mexicanos. Guia de refrencia rapida diagnostico y tratamient de artritis reumatoide del adulto. Distrito Federal, Mexico: Gobierno Federal de Estados Unidos Mexicanos; 2012. http://www.cenetec.salud.gob.mx/descargas/gpc/CatalogoMaestro/195_ARTRITIS_REUMATOIDE/artritis_reumatoide_RR_CENETEC.pdf. Accessed October 4, 2013
- Citera G, Ficco HM, Alamino RS, et al. Work disability is related to the presence of arthritis and not to a specific diagnosis. Results from a large early arthritis cohort in Argentina. Clin Rheumatol 2015;34:929-33
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7.
- Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis 2010;4:28-62
- Birnbaum HG, Pike C, Banerjee R, et al. Changes in utilization and costs for patients with rheumatoid arthritis, 1996 to 2006. Pharmacoeconomics 2012;30:323-36.
- Singh JA, Christensen R, Wells GA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2009:CD007848
- Sprakes MB, Ford AC, Suares NC, et al. Costs of care for Crohn's disease following the introduction of infliximab: a single-centre UK experience. Aliment Pharmacol Ther 2010;32:1357-63
- Sussman DA, Kubiliun N, Mulani PM, et al. Comparison of medical costs among patients using adalimumab and infliximab: a retrospective study (COMPAIRS). Inflamm Bowel Dis 2012;18:2043-55
- Binion DG, Louis E, Oldenburg B, et al. Effect of adalimumab on work productivity and indirect costs in moderate to severe Crohn’s disease: a meta-analysis. Can J Gastroenterol 2011;25:492-6
- World Bank World Development Indicators. Washington, DC: World Bank; 2013. http://databank.worldbank.org. Accessed August 6, 2013
- International Monetary Fund. World Economic Outlook Database, October 2014. Projected 2013 population. Washington, DC, USA. http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/weoselgr.aspx. Accessed October 3, 2014
- WHO Global Health Expenditures Database. Geneva, Switzerland: WHO; 2013 and 2014. http://apps.who.int/nha/database/PreDataExplorer.aspx?d=1. Accessed February 7, 2013 for Brazil; August 6, 2013, for Argentina; October 3, 2014 for Mexico
- WHO Global Health Expenditures Database. Geneva, Switzerland: WHO; 2014. http://apps.who.int/nha/database/ViewData/Indicators/en. Accessed November 13, 2014 for Colombia
- National Institute of Statistics and Census of Argentina (INDEC). Index of Salaries and Coefficient of Salary Variation. Buenos Aires, Argentina: INDEC; 2013. http://www.indec.gov.ar/nivel4_default.asp?id_tema_1=4&id_tema_2=31&id_tema_3=61. Accessed August 26, 2013
- International Monetary Fund. World Economic Outlook Database, April 2013. Projected 2012 population. Washington, DC, USA. http://www.imf.org/external/pubs/ft/weo/2013/01/weodata/weoselgr.aspx. Accessed August 5, 2013
- Banco Central do Brasil. Foreign exchange and wage indicators. Brasilia, Brazil: Banco Central do Brasil; 2014. http://www.bcb.gov.br/pec/Indeco/Ingl/ie5-35i.xls. Accessed October 3, 2014
- ILO Global Wage Database - Mexico National Institute of Statistics and Geography. ILO Global Wage Database - Mexico National Institute of Statistics and Geography. Geneva, Switzerland: International Labor Organization; 2013.http://www.ilo.org/travail/areasofwork/wages-and-income/WCMS_142568/lang–en/index.htm. Accessed September 30, 2013
- Culpepper Salary Budget Survey Data Tables 2012–2013. Culpepper and Associates Inc. (publisher and author).Alpharetta, GA: Culpepper and Associates Inc.; 2013. http://www.culpepper.com/dl.asp?f=/surveys/practices/SalaryBudget/20122013/Culpepper20122013SalaryBudgetSurveyDataTables.xlsx. Accessed May 20, 2014
- Culpepper Salary Budget Survey Data Tables 2013–2014. Culpepper and Associates Inc. (publisher and author). Alpharetta, GA: Culpepper and Associates Inc.; 2014. http://www.culpepper.com/dl.asp?f=/surveys/practices/SalaryBudget/20122013/Culpepper20122013SalaryBudgetSurveyDataTables.xlsx. Accessed May 20, 2014 and November 13, 2014
- National Institute of Statistics and Census of Argentina (INDEC). Serie histórica del Indice de Precios al Consumidor (IPC) en el Gran Buenos Aires. Buenos Aires, Argentina: INDEC; 2013. http://www.indec.mecon.ar/informacion-de-archivo.asp Accessed August 26, 2013.
- World Bank Population Data. Washington, DC: World Bank; 2015. http://data.worldbank.org/region/LAC; http://data.worldbank.org/country/argentina. Accessed September 15, 2015
- International Monetary Fund. World Economic Outlook Database, April 2013. Projected 2012 GDP current prices, national currency. Washington, DC. http://www.imf.org/external/pubs/ft/weo/2013/02/weodata/weoselgr.aspx. Accessed August 5, 2013
- Catay E, Del Cid CC, Narváez L, et al. Cost of rheumatoid arthritis in a selected population from Argentina in the prebiologic therapy era. Clinicoeconomics and Outcomes Res 2012;4:219-25
- Chermont GC, Kowalski SC, Ciconelli RM, et al. Resource utilization and the cost of rheumatoid arthritis in Brazil. Clin Exp Rheumatol 2008;26:24-31
- Instituto Brasiliero de Geografia e Estatistica. IPCA Index. Rio de Janeiro, Brazil: Instituto Brasiliero de Geografia e Estatistica; 2013. http://www.ibge.gov.br/english/estatistica/indicadores/precos/inpc_ipca/defaultseriesHist.shtm. Accessed February 20, 2013
- Montoya N, Gomez L, Velez M, et al. Costos directos del tratamiento de pacientes con artritis reumatoide en Medellín, Colombia. Rev Colomb Reumatol 2011;18:26-33
- Banco de la Republic Colombia. Indice de precios al consumidor Bogota, Colombia, 2013. http://banrep.gov.co/series-estadisticas/see_precios_ipc.htm. Accessed August 6, 2013
- Álvarez-Hernández E, Peláez-Ballestas I, Boonen A, et al. Catastrophic health expenses and impoverishment of households of patients with rheumatoid arthritis. Reumatol Clin 2012;8:168-73
- Instituto Nacional de Estadística y Geografía. Índices de Precios al Consumidor. INPC Nacional. Aguascalientes, Mexico, 2013. http://www.inegi.org.mx/est/contenidos/proyectos/inp/inpc.aspx. Accessed February 5, 2013
- de Azevedo AB, Ferraz MB, Ciconelli RM. Indirect costs of rheumatoid arthritis in Brazil. Value Health 2008;11:869-77
- Sokka T, Kautiainen H, Pincus T, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA Study. Arthritis Res Ther 2010;12:R42
- Piedrahita H. Costs of work-related musculoskeletal disorders (MSDs) in developing countries: Colombia case. Int J Occup Saf Ergon 2006;12:379-86
- Araujo G, Fonseca M. Cost of patient care in patients with Crohn’s disease in Brazil: public health perspective. Value Health 2007;10:A149
- Morais AD, Pereira ML, Paloni EDMP, et al. Real life treatment cost of rheumatoid arthritis, psoriasis, Crohn’s disease and ulcerative colitis in the Brazilian private health care system. Poster presented at: ISPOR 17th Annual International Meeting; June 2–6, 2012; Washington, DC, USA
- Loomes DE, Teshima C, Jacobs P, et al. Health care resource use and costs for Crohn's disease before and after infliximab therapy. Can J Gastroenterol 2011;25:497-502
- Longobardi T, Jacobs P, Bernstein CN. Work losses related to inflammatory bowel disease in the United States: results from the National Health Interview Survey. Am J Gastroenterol 2003;98:1064-72
- Birnbaum H, Pike C, Kaufman R, et al. Employer model of workplace impacts of anti-TNF therapy for rheumatoid arthritis. J Occup Environ Med 2009;51:1167-76
- Augustsson J, Neovius M, Cullinane-Carli C, et al. Patients with rheumatoid arthritis treated with tumour necrosis factor antagonists increase their participation in the workforce: potential for significant long-term indirect cost gains (data from a population-based registry). Ann Rheum Dis 2010;69:126-31
- Feagan BG, Reilly MC, Gerlier L, et al. Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate-to-severe Crohn's disease in the PRECiSE 2 study. Aliment Pharmacol Ther 2010;31:1276-85
- Senna, ER, De Barros ALP, Silva EO, et al. Prevalence of rheumatic diseases in Brazil: A study using the COPCORD approach. J Rheumatol 2004;31:594-7
- Titton DC, Silveira IG, Louzada-Junior P, et al. Brazilian biologic registry: BiobadaBrasil implementation process and preliminary results. Rev Bras Reumatol 2011;51:152-60
- International Monetary Fund. World Economic Outlook Database, October 2012. Projected 2012 population. Washington, DC, 2012. http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/weoselgr.aspx. Accessed February 20, 2013
- International Monetary Fund. World Economic Outlook Database, October 2012. Projected 2012 GDP current prices, national currency. Washington, DC, 2012. http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/weoselgr.aspx. Accessed February 20, 2013
- Santos-Moreno P, Gonzalez-Malaver F, Fandiño C, et al. Improvement in activity levels short term in a large cohort of patients with rheumatoid arthritis implementing the recommendations: target to treat. Rev Colomb Reumatol 2011;18:2(Suppl):61
- International Monetary Fund. World Economic Outlook Database, October 2014. Projected 2013 GDP, national currency. Washington, DC, 2014. http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/weoselgr.aspx. Accessed October 3, 2014
- Feagan BG, Bala M, Yan S, et al. Unemployment and disability in patients with moderately to severely active Crohn's disease. J Clin Gastroenterol 2005;39:390-5
- Hay JW, Hay AR. Inflammatory bowel disease: costs-of-illness. J Clin Gastroenterol 1992;14:309-17
- Gibson TB, Ng E, Ozminkowski RJ, et al. The direct and indirect cost burden of Crohn's disease and ulcerative colitis. J Occup Environ Med 2008;50:1261-72
- Yu AP, Cabanilla LA, Wu EQ, et al. The costs of Crohn's disease in the United States and other Western countries: a systematic review. Curr Med Res Opin 2008;24:319-28
- Ministerio da Saude do Brasil. Estudos de avaliacao economica de technologias em saude [Economic evaluation studies of technologies in health]. Brasilia, Brazil, 2009. http://www.ispor.org/PEguidelines/source/Economic-Evaluation-Guidelines-in-Brazil-Final-Version-2009.pdf. Accessed May 7, 2013 and October 3, 2014. Portuguese
- Ministerio de la Proteccion Social Republica de Colombia. Guía Metodológica para la elaboración de Guías de Atención Integral en el Sistema General de Seguridad Social en Salud colombiano, May Bogota, Colombia, 2010. http://www.minsalud.gov.co/salud/Documents/Gu%C3%ADa%20Metodol%C3%B3gica%20para%20la%20elaboraci%C3%B3n%20de%20gu%C3%ADas.pdf. Accessed August 6, 2013
- Augustovski F, Garay OU, Pichon-Riviere A, et al. Economic evaluation guidelines in Latin America: a current snapshot. Expert Rev Pharmacoecon Outcomes Res 2010;10:525-37
- Spindler A, Bellomio V, Berman A, et al. Prevalence of rheumatoid arthritis in Tucumán, Argentina. J Rheumatol 2002;29:1166-70
- Scublinsky D, Venarotti H, Citera G, et al. The prevalence of rheumatoid arthritis in Argentina: a capture-recapture study in a city of Buenos Aires province. J Clin Rheumatol 2010;16:317-21
- Cardiel MH, Latin American Rheumatology Associations of the Pan-American League of Associations for Rheumatology (PANLAR); Grupo Latinoamericano de Estudio de Artritis Reumatoide (GLADAR). First Latin American position paper on the pharmacological treatment of rheumatoid arthritis. Rheumatology (Oxford) 2006;45(Suppl 2):ii7-ii22
- Peláez-Ballestas I, Sanin LH, Moreno-Montoya J, et al. Epidemiology of the rheumatic diseases in Mexico. A study of 5 regions based on the COPCORD methodology. J Rheumatol Suppl 2011;86:3-8
- Correa M, Lencina V, del Moral R, et al. Impacto de las Guías de Práctica Clínica para el Tratamiento de la Artritis Reumatoidea. Rev Arg Reumatol 2012;23:18-23
- Caballero-Uribe V, Londoño JD, Chalem P. Tratamiento de la artritis reumatoide en Colombia. Revista Colombiana de Reumatología 2002;9:242-50
- Economic Commission for Latin America and the Caribbean (ECLAC). Total population 2013. Santiago, Chile, 2013. http://interwp.cepal.org/anuario_estadistico/anuario_2013/en/index.asp. Accessed April 1, 2014
- Goycochea-Robles MV, Arce-Salinas CA, Guzmán-Vázquez S, Cardiel-Ríos MH. Prescription rheumatology practices among Mexican specialists. Arch Med Res 2007;38:354-9
- de Jesús Yepes Barreto I, Carmona R, Díaz F, et al. Prevalence and demographic characteristics of inflammatory bowel disease in Cartagena, Colombia. Rev Col Gastroenterol 2010:25:107-11
- Miltenburger C, Munkombwe M, Lekander I. A survey of barriers to treatment access in rheumatoid arthritis in major Latin American Countries – Argentina, Brazil and Mexico. i3 innuvo, Medford, MA, 2010. http://www.comparatorreports.se/LA%20RA%20barrier%20report_FINAL.pdf. Accessed May 9, 2016