Abstract
Objective: To evaluate cost-effectiveness of brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma who have received autologous stem cell transplantation, from a Scottish healthcare payer perspective.
Methods: A Microsoft Excel-based partitioned survival model comprising three health states (progression-free survival [PFS], post-progression survival, and death) was developed. Relevant comparators were chemotherapy with or without radiotherapy (C/R) and C/R with intent to allogeneic hematopoietic stem cell transplantation (alloSCT). Data were obtained from the pivotal phase II single-arm trial in 102 patients (SG035-0003; NCT00848926), a systematic literature review and clinical expert opinions (where empirical evidence was unavailable). PFS and overall survival for brentuximab vedotin were estimated using 5-year follow-up data from SG035-0003, and extrapolated using event rates observed for comparator treatments from published survival data. Resource use included drug acquisition and administration; alloSCT; treatment of adverse events; and long-term follow-up. Deterministic and probabilistic sensitivity analyses were conducted to evaluate the impact of uncertainty.
Results: In the base case, the incremental cost-effectiveness ratio (ICER) for brentuximab vedotin was £38,769 per quality-adjusted life year (QALY) vs C/R, whereas C/R with intent to alloSCT was dominated by brentuximab vedotin. ICERs for brentuximab vedotin generated by the deterministic sensitivity analysis ranged between £32,000–£54,000 per QALY. Including productivity benefits reduced the ICER to £28,881 per QALY.
Limitations: Limitations include lack of comparative data from this single arm study and the heterogeneous population. Inconsistent baseline characteristic reporting across studies prevented complete assessment of heterogeneity and the extent of potential bias in clinical and cost-effectiveness estimates.
Conclusions: Although the base case ICER is above the threshold usually applied in Scotland, it is relatively low compared with other orphan drugs, and lower than the ICER generated using a previous data cut of SG035-0003 that informed a positive recommendation from the Scottish Medicines Consortium, under its decision-making framework for assessment of ultra-orphan medicines.
Introduction
Classical Hodgkin lymphoma (HL) is a rare cancer of the immune system that usually affects adults aged 20–24 years, followed by another peak in adults aged ≥70 yearsCitation1. Data from the Scottish Cancer Registry indicate that there were 161 new cases of HL diagnosed in Scotland in 2013Citation2. In 2009, HL was estimated to affect approximately 1 in 10,000 people in the European Union (EU), which is well below the threshold (5 people in 10,000) for designation as an orphan indicationCitation3.
Chemotherapy with or without radiotherapy (C/R) is the standard of care for limited-stage HLCitation4. However, ∼15% of patients are refractory or relapse following frontline treatmentCitation5. Standard treatment for relapsed or refractory (R/R) HL is high-dose chemotherapy followed by autologous stem cell transplant (ASCT)Citation4, but data from a number of studies suggest that up to 50% of R/R HL patients will ultimately relapse after ASCTCitation6. Patients who fail ASCT have a very poor prognosis, with a median overall survival (OS) of 2.4 years, which falls to only 1.2 years in patients who relapse within 1 year of ASCTCitation7,Citation8.
Treatment guidelines and observational data indicate that, historically, the most commonly used treatment in these patients was multi-agent C/R, which may be followed by allogeneic stem cell transplant (alloSCT) in eligible patientsCitation4,Citation9. However, these approaches are increasingly being challenged by the introduction of novel agents.
Brentuximab vedotin (ADCETRIS1, Seattle Genetics, Inc., Bothell, WA) is an antibody–drug conjugate with a distinct mechanism of action that results in selective apoptosis of CD30-expressing tumor cellsCitation10. CD30 is consistently expressed in patients who are refractory to multi-agent chemotherapy, regardless of prior transplant statusCitation11. In the US, brentuximab vedotin received accelerated approval by the Food and Drug Administration for the treatment of patients with classical HL after failure of ASCT or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates, and is approved for classical HL at high risk of relapse or progression post-ASCTCitation10. In addition, brentuximab vedotin has received conditional approval from the European Medicines Agency for the treatment of adult patients with R/R CD30-positive HL following ASCT; or following failure of at least two prior therapies where ASCT or multi-agent chemotherapy is not a treatment optionCitation11. The approval of brentuximab vedotin in HL was based on data from a pivotal phase II, open-label, single-arm, multi-center study in 102 patients with R/R HL after ASCT (SG035-0003; NCT00848926). Eligible patients were aged ≥12 years with R/R HL after ASCT, and patients with prior alloSCT were ineligible. Patients received brentuximab vedotin 1.8 mg/kg IV once every 3 weeks for up to 16 cyclesCitation12–15. The objective response rate (ORR) per the independent review facility (IRF) was 75%; 34% of patients achieved a complete remission (CR) and 40% achieved a partial remission (PR)Citation15. Follow-up data from SG035-0003, based on a median follow-up of 32.7 months, indicate a median OS of 40.5 months, with an estimated 3-year survival rate of 54%Citation13. The median OS was also confirmed in the 5-year follow-up data from this study, based on a median follow-up of 35.1 months (range =1.8–72.9 months)Citation12. This finding compares favorably with the median OS reported in the literature for such patients (∼1–2 years)Citation7,Citation8.
Recommendations for the use of new therapies by some health technology assessment (HTA) bodies—such as the UK National Institute for Health and Care Excellence (NICE) and Scottish Medicines Consortium (SMC)—depend not only on the efficacy and safety of such treatments but also their cost-effectiveness, expressed in terms of an incremental cost-effectiveness ratio (ICER)Citation16. In 2015, the SMC made a positive recommendation for brentuximab vedotin in patients with R/R HL who have received ASCTCitation17, which was informed by 3-year follow-up data from SG035-0003. Here, we present the cost-effectiveness analysis that informed this recommendation, updated with 5-year follow-up data from SG035-0003.
Patients and methods
Population
In line with SG035-0003, the model evaluates brentuximab vedotin in patients who have failed high-dose chemotherapy and ASCT. Patients entered the trial within 1 year of ASCT relapse in 71% of cases. Of the 56% patients who had received post-ASCT treatment, 42% were refractory to their last treatment and 14% had relapsed after their last treatment. Patients had received one, two, and three or more post-ASCT treatments in 20%, 17%, and 20% of cases, respectively. Clinicians suggested that brentuximab vedotin may, therefore, be used in three patient groups post-ASCT failure, which the SG035-0003 study population are split across: (1) first-line post-ASCT relapse in patients not eligible for alloSCT (those aged >60–70 years or with significant comorbidities); (2) first-line post-ASCT relapse, as an induction treatment for alloSCT in patients eligible for an alloSCT; and (3) second or subsequent line post-ASCT relapse.
Comparators
Treatment guidelines do not recommend a specific chemotherapy regimen for post-ASCT relapseCitation4; therefore, chemotherapy consisting of a range of regimens was considered as a composite comparator. This was considered reasonable, as little is known about their relative efficacy, and all the chemotherapies considered are associated with similar costs.
The cost-effectiveness analysis compared brentuximab vedotin with C/R and C/R with intent to alloSCT. Analyses are presented with and without the latter comparator, as for some patients this treatment would be precluded at baseline due to age or comorbidities.
Model structure
A partitioned survival model was developed using Microsoft Excel 2010 to project the costs and outcomes for adult patients with R/R CD30-positive HL following ASCT failure over a lifetime (40-year) time horizon. Three health states were included: progression-free survival (PFS); post-progression survival (PPS); and death. The proportion of patients in the PFS state over time was estimated directly from the PFS curves, and the proportion of patients in the PPS state was estimated as the difference between the OS curve and the PFS curve. Costs and quality-adjusted life years (QALYs) were accrued according to the proportion of patients in the PFS and PPS states over time.
The analysis was conducted from the perspective of the National Health Service (NHS) in Scotland. The year of the cost analysis was 2013. For accuracy, costs and outcomes were evaluated on a daily basis and were discounted at a rate of 3.5% per annumCitation18. The model estimates these outcomes for three cohorts: (1) patients who received only brentuximab vedotin; (2) patients who received only C/R; and (3) patients who received only alloSCT.
Not all patients intended to receive alloSCT actually receive a transplant. In order to generate outcomes for the C/R with intent to alloSCT comparator, the long-term outcomes associated with the C/R and alloSCT cohorts were weighted according to the estimated proportion of patients eligible for alloSCT who ultimately receive alloSCT. In the base case, this weighting was based on data from a retrospective study in which 56% of 185 patients intended for transplant (as indicated by human leukocyte antigen [HLA] typing within 30 days of ASCT relapse) were actually allograftedCitation19.
Model inputs
Clinical data
Data from the SG035-0003 study were used to inform the efficacy and safety of brentuximab vedotinCitation12,Citation13. The efficacy and safety of the comparator treatments was informed by a systematic review, as discussed in Supplementary Data 1. Patient and disease characteristics for each data source that informed the clinical outcomes in the model are shown in . Demographic and clinical characteristics were similar across the three study populations.
Table 1. Demographic and clinical characteristics of patients enrolled in clinical studies informing the cost-effectiveness model.
Progression-free survival
PFS for brentuximab vedotin was estimated based on the observed PFS Kaplan-Meier curve up to the maximum of 6.1 years of follow-up of SG035-0003Citation12,Citation13. Investigator-assessed data rather than IRF were used in the base case, as few studies in the available literature for the comparators include an independent assessment.
For C/R, PFS was estimated using data from SG035-0003 patients collected during the period following the most recent prior post-ASCT therapy. These data were selected in preference to data from the literature which were from small studies and unlikely to be representative of the heterogeneous set of regimens received by these patients. Moreover, use of self-control data may have controlled for some of the inherent bias associated with conducting naïve comparisons. Patients in the alloSCT cohort were assumed to remain progression-free for the average chemotherapy duration period (3.9 months), calculated based on the mean number of cycles reported in the literature for each of the modeled chemotherapy regimensCitation20–26. Thereafter, PFS was assumed to follow the experience of patients in the European Group for Blood and Marrow Transplantation (EBMT) registry, as described in a study of reduced-intensity conditioning (RIC) alloSCT in 285 HL patients that captures data across the main European countries. This study was selected as it is the largest study of RIC alloSCT identified by the systematic review and is based on the EBMT registry which is thought to capture the majority of transplants conducted in the main European countries. Follow-up data for 5.2 years are available from this studyCitation27.
To generate lifetime estimates of costs and QALYs, extrapolation of the PFS data for the brentuximab vedotin and alloSCT cohorts was necessary, as not all patients still being followed-up had experienced an event. For alloSCT, EBMT registry data suggest that the risk of progression stabilized during the last ∼40 months of follow-upCitation27. Therefore, a constant risk of progression beyond the 5.2-year period described above was assumed for this cohort. For brentuximab vedotin, the risk of progression following the maximum 6.1-year follow-up in the SG035-0003 studyCitation12 was assumed to be equal to alloSCT, as limited data on PFS in non-alloSCT treated patients are available.
Overall survival
OS for brentuximab vedotin was estimated directly from SG035-0003 study data, based on the Kaplan-Meier curve for the maximum 6.1-year follow-up periodCitation12. However, in the base case analysis, the OS Kaplan-Meier curve was only used up to 296 weeks due to rapid loss to follow-up after this time point.
Data for the other comparators were taken from another EBMT observational cohort study (reported by Martinez et al.Citation9 and MartinezCitation28) that provided 6-year Kaplan-Meier OS curves for patients who receive C/R (n = 294), or RIC or myeloablative alloSCT (n = 133), stratified according to whether patients had 0, 1, or ≥2 of the following risk factors: relapse within 6 months of ASCT; Ann Arbor Stage IV disease at ASCT relapse; bulky disease; age >50 years; and poor performance statusCitation9. This study was selected as it is the largest available and allows for some level of adjustment for differences in the population with regards to the SG035-0003 study using the published data.
The OS Kaplan-Meier curves were adjusted for the risk-factor distribution in the SG035-0003 study by weighting the EBMT data to reflect the proportion of patients with 0, 1, and ≥2 risk factors in SG035-0003. The adjusted curves were used directly for the maximum follow-up period of 71.79 months in the Martinez et al.Citation9 study.
The probability of death beyond the 71.79 months of follow-up was estimated based on an OS curve that represented a weighted average of the adjusted survival curves for C/R and RIC alloSCT (weighted by the proportion of patients receiving each treatment in the EBMT study)Citation9. Inspection of the probability of death associated with the resulting weighted survival curve during each 6-month period indicated that the probability of death was relatively constant from 30–66 months. In the base case analysis, the probability of death observed over this period was, therefore, applied to the model as a constant rate to patients in the alloSCT and C/R-only cohorts beyond the maximum follow-up in the EBMT studyCitation9. As a sensitivity analysis, a Weibull survival model was fitted to the aggregate data to predict long-term OS. Beyond the SG035-0003 study period (6.08 years), brentuximab vedotin treatment was assumed to be associated with a hazard ratio (HR) of 1.00 compared to the other comparators (i.e. patients in the brentuximab vedotin cohort were considered to experience the same probability of death as patients receiving the other comparators).
Adverse events
Adverse events (AEs) were included in the model if they satisfied at least one of the following criteria: grade 3–4 event that occurred for any comparator in ≥5% of patients (or cycles if percentage of patients not reported); or grade 1–2 event occurring in ≥20% of patients (or cycles). AEs were also required to have material implications for cost or quality-of-life. Event rates for brentuximab were taken from the SG035-0003 study and rates for the comparators were taken from the literatureCitation12,Citation21,Citation24,Citation25,Citation29–31 (Supplementary Data 2).
Resource use and costs
A detailed summary of costs is presented in Supplementary Data 2. Resource use included drug acquisition and administration, concomitant medications, radiotherapy, alloSCT, AEs, and long-term follow-up. Drug costs were taken from British National Formulary, March 2014Citation32. Unit costs for all other resources were sourced from the NHS Reference Costs 2012–2013Citation33. All drug cost calculations were made assuming vial wastage (as patient numbers in each center would be likely to be too low to allow any vial sharing).
Patients receiving brentuximab vedotin required a single infusion per cycle to administer the drug. Brentuximab vedotin was supplied as 50 mg vials (£2500 per vial) and was administered in the SG035-0003 study on an outpatient basis at a dose of 1.8 mg/kg, every 21 days for an average of 9.7 cycles. The reported relative dose intensity (RDI) was 93.5%. The weight distribution in the SG035-0003 study was used to estimate the number of vials required per patient, based on the per-kg dosage, RDI, and patients’ weights.
The cost of chemotherapy was estimated based on clinical expert opinion regarding the frequency of use of different treatment regimens in the UK. Scottish specialists confirmed similar usage in Scotland to the rest of the UK. In addition, 10% of patients were assumed to require radiotherapy as adjunct to their chemotherapy based on clinical input. This was allocated as a one-off cost at the beginning of treatment.
Delivery of chemotherapy was assumed to occur on an outpatient basis for all comparators. In addition, the following concomitant medications were captured in the model: anti-emetics for all chemotherapy and brentuximab vedotin treatments, and immunosuppressive treatment for patients who receive an alloSCT.
A weighted cost of alloSCT (£108,052) for sibling donors and volunteer unrelated donors was provided by the Bone Marrow Transplant Unit at the Beatson West of Scotland Cancer Centre (WoSCC), Glasgow, based on the assumption that two-thirds of alloSCTs undertaken in Scotland involve an unrelated donor.
Due to the lack of data available, assumptions on AE-related resource use were obtained from clinical experts in the UK (Supplementary Data 2).
Follow-up care before progression comprised outpatient visits, blood counts, biochemistry, and computed tomography (CT) scans. Corresponding resource use was estimated based on clinical opinion, and was stratified according to whether patients received alloSCT and were on or off treatment. In the “post-progression”’ state, patients were assumed to experience a one-off cost of treatment on progression (equal to the chemotherapy acquisition and administration cost, as well as the total cost of AEs associated with standard chemotherapy). The long-term follow-up cost for post-progression patients was assumed to be equal to the cost for the on-treatment period for chemotherapy.
Health-related quality-of-life
Utility data were taken from a vignette studyCitation34 that elicited time trade-off valuations from 100 members of the general public in the UK. Disease state vignettes (for CR, PR, stable disease [SD], and progressive disease [PD]) were developed based on a review of the literature and on patient and clinician interviews, to represent the experience of both post-ASCT HL patients’ and patients with R/R systemic anaplastic large cell lymphomaCitation34.
In order to capture the impact of the different response rates on quality-of-life, the utility level in the PFS state was weighted according to the proportion of patients in each response category for each comparator (Supplementary Data 2). For brentuximab vedotin, investigator-assessed response rates were used. For C/R, response data were taken from the response to prior post-ASCT treatment in SG035-0003. For RIC alloSCT, response rates at 100-days post-transplant were obtained from the report by Robinson et al.Citation27, and applied from the time of transplant until progression. Prior to 100-days post-transplant, alloSCT patients were assumed to experience the same response rates as when treated with chemotherapy.
For AEs, utility decrements were combined with estimated event durations to generate a QALY decrement for each event (Supplementary Data 2). These effects were assumed to be independent for each event, hence were modeled additively. Where data were not collected from the vignette study, utility decrements obtained from similar studies in other cancers were appliedCitation34–36, and event durations were based on clinical input.
Sensitivity analyses
Extensive deterministic sensitivity analyses were conducted to evaluate the robustness of the cost-effectiveness model. These included assuming a level of “catch up” (HR >1.00) between brentuximab vedotin and the comparators for PFS and OS after the SG035-0003 study follow-up; varying the relative effects of brentuximab vedotin vs the comparators for PFS and OS over lifetime; use of IRF-assessed response and PFS data for brentuximab vedotin; use of the maximum follow-up from SG035-0003 for OS for brentuximab vedotin; and varying the cost of alloSCT.
A scenario analysis was also conducted, incorporating the potential productivity benefits of treatment. Based on expert clinical opinion, the productivity scenario analysis assumed that 40%, 15%, and 10% of patients in the CR, PR, and SD health states, respectively, return to work in the PFS state. This corresponded to a productivity benefit of £18,172, £2,567, and £19,034 for brentuximab vedotin, C/R, and alloSCT, respectively. Patients were not able to return to work for 1 year following alloSCT and could not return to work in the PPS state. For each year patients are able to return to work, they accrue a productivity benefit (the average annual wage) of £26,522Citation37. Additional detail on the corresponding inputs for the productivity scenario analyses are presented in Supplementary Data 3, and response rates in the PFS state for each comparator are presented in Supplementary Data 2.
A probabilistic sensitivity analysis (PSA) was also conducted by assigning distributions to all input parameters and randomly sampling from these distributions over 5000 Monte Carlo simulations to calculate the uncertainty in the cost and health outcome measures.
Results
Clinical outcomes
Long-term outcomes predicted by the model show PFS for brentuximab vedotin that is superior to that observed with C/R and, by a lesser margin, to that observed following C/R with intent to alloSCT. Brentuximab vedotin also showed an OS advantage over C/R with or without intent to alloSCT. These survival advantages were observed within the trial period (); however, the majority of the OS (and to some extent the PFS) gain was driven by the period beyond the SG035-0003 study follow-up ().
Figure 1. Kaplan-Meier estimates of PFS and OS, by comparator. (a) Brentuximab vedotin: PFS (investigator-assessed) and OS were taken from SG035-0003Citation12, measured from trial entry. (b) C/R: PFS was taken from SG035-0003 for the most recent prior post-ASCT therapy (n = 56), measured from trial entryCitation12; OS was taken from Martinez et al., 2013Citation9 for C/R, measured from ASCT failure and adjusted for case mix. (c) AlloSCT: PFS was taken from Robinson et al., 2009Citation27 for RIC alloSCT, measured from ASCT failure; OS was taken from Martinez et al., 2013Citation9 for RIC and myeloblative alloSCT, measured from ASCT failure and adjusted for case mix. alloSCT, allogenic stem cell transplant; ASCT, autologous stem cell transplant; C/R, chemotherapy ± radiotherapy; PFS, progression-free survival; OS, overall survival; RIC, reduced-intensity conditioning.
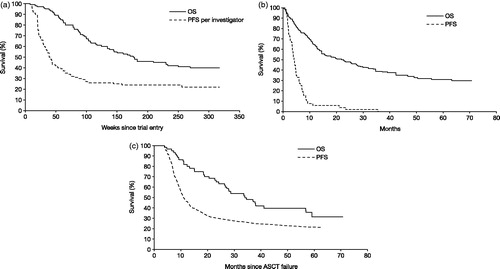
Figure 2. Long-term extrapolations of PFS and OS, by comparator. (a) PFS: 0–6.08 years, brentuximab vedotin SG035-0003 dataCitation12 were compared with self-control and Robinson et al., 2009Citation27 data; 6.08 years–lifetime, all comparators were assumed to have the same constant risk of progression. (b) OS: 0–5.67 years, brentuximab vedotin SG035-0003 dataCitation12 were compared directly with Martinez et al., 2013Citation9 data; 5.67–6.0 years, brentuximab vedotin dataCitation12 were extrapolated with a hazard ratio of 1.0 applied to the Martinez et al., 2013Citation9 hazard; 6.0–lifetime, all comparators were assumed to have the same constant risk of death. alloSCT, allogenic stem cell transplant; C/R, chemotherapy ± radiotherapy; OS, overall survival; PFS, progression-free survival.
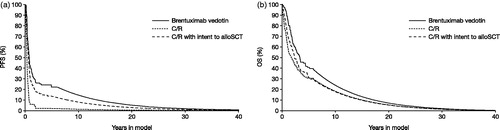
Brentuximab vedotin-treated patients accrued total QALYs of 3.36, yielding incremental QALYs of 1.58 vs C/R and 0.85 vs C/R with the intent to alloSCT. QALY gains for brentuximab vedotin and C/R with intent to alloSCT compared to C/R were both driven by gains in the PFS state. The gains associated with C/R with intent to alloSCT vs C/R only were offset to some extent by the QALY decrement associated with alloSCT-related AEs.
Costs
Patients treated with brentuximab vedotin incurred total costs of £88,572, yielding incremental costs of £61,179 vs C/R and –£6,421 vs C/R with intent to alloSCT (). The incremental costs of brentuximab vedotin compared to C/R were driven by the acquisition cost of brentuximab vedotin treatment. The incremental costs of C/R with intent to alloSCT were driven by both the cost of transplantation and the higher cost of AEs.
Table 2. Discounted life years (LYs), QALYs and costs by resource category.
Cost-effectiveness
For some patients alloSCT would be precluded at baseline due to age or comorbidities; therefore, analyses were conducted including and excluding C/R with intent to alloSCT from the comparator set. The ICER for brentuximab vedotin was £38,769 per QALY gained vs C/R when excluding C/R with intent to alloSCT (). For patients eligible for alloSCT, C/R with intent to alloSCT was dominated by brentuximab vedotin. The prior cost-effectiveness estimate is, therefore, applicable to all patients, regardless of eligibility for alloSCT. The same pattern of results was observed in the analysis of cost per life-year (LY), in which the ICER for brentuximab vedotin was £47,593 per LY gained vs C/R.
Table 3. Base case cost-effectiveness.
Sensitivity analyses
When considering all comparators, the ICER for brentuximab vedotin ranged between £38,000–£50,000 per QALY for the majority of the analyses (). The following scenarios yielded ICERs above or below these boundaries: assuming 18% of patients receiving brentuximab vedotin go on to receive alloSCT (£53,030 per QALY), use of IRF data for PFS and response to brentuximab vedotin (£51,937 per QALY), assuming all patients achieve a CR utility until progression (£34,097 per QALY), and assuming all patients receive the most expensive chemotherapy regimen (£32,998). C/R with intent to alloSCT remained dominated or extended dominated in all analyses. Hence, the ICERs for brentuximab vedotin are applicable, regardless of eligibility for alloSCT.
Table 4. Deterministic sensitivity analyses.
The alloSCT cohort had the greatest productivity benefit (–£19,034), which was driven by its superior response profile (Supplementary Data 3). When accounting for the proportion of patients who proceed to transplant in the C/R with intent to alloSCT comparator, brentuximab vedotin has the greatest productivity benefit (–£18,172) compared to C/R (–£2,567) and C/R with intent to alloSCT (–£11,824). Incorporating these productivity benefits reduced the ICER for brentuximab vedotin to £28,881 per QALY vs C/R.
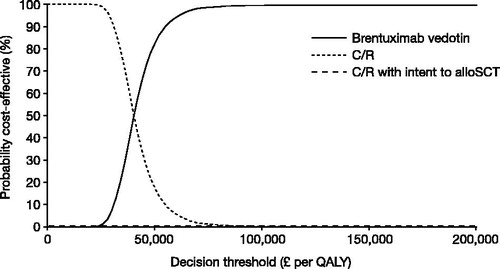
The probability that brentuximab vedotin is cost-effective depends on the cost-effectiveness threshold. shows the probability that brentuximab vedotin is cost-effective at a range of cost-effectiveness thresholds.
Discussion
The base case ICER for brentuximab vedotin estimated by this analysis is £38,769 per QALY compared to C/RCitation17. This result was robust to a wide range of scenario analyses, including analyses assuming that brentuximab vedotin is associated with higher rates of PFS or OS events than the comparators beyond the trial follow-up. Only two scenarios increased the ICER to above £50,000 per QALY. For patients eligible to receive alloSCT, C/R with intent to alloSCT was dominated by brentuximab vedotin.
This evaluation compared brentuximab vedotin with two treatments recommended for use in patients with R/R HL post-ASCT over a lifetime time horizon, based on the best available evidence for these treatments. Data for brentuximab vedotin were taken from the SG035-0003 study which informed expedited approval from the regulatory authorities in Europe and the US for patients with R/R HL post-ASCT. Data for the comparators were taken from a combination of SG035-0003 and EBMT observational cohort studies, the latter of which is the largest available study of chemotherapy with or without radiotherapy in R/R HL. In addition, the EBMT study provided information on risk factors, which allowed the observational data to be adjusted according to baseline prognosis, in line with SG035-0003 data.
Drugs for rare diseases (orphan drugs) are often expensive and do not prove to be cost-effective based on standard methods of HTA, which may mean that funding and patient access are limited. However, the cost-effectiveness estimate for brentuximab vedotin (£38,769 per QALY gained) is relatively low compared to other orphan drugs that are recommended in the UK, examples of which include ipilimumab for advanced/metastatic melanoma (at ∼£42,200 per QALY gained), and pemetrexed as maintenance therapy in non-small cell lung cancer (between £41,300–£51,000 per QALY gained)Citation38,Citation39. The SMC made its positive recommendation for brentuximab vedotin in 2015 under its decision-making framework for the assessment of ultra-orphan medicines. The associated criteria include the nature of the condition, the impact of the new technology, value for money, and impact beyond direct health benefits and on specialist servicesCitation17. In addition, current HTA methodology by NICE in the UK effectively recommends a higher decision threshold for end-of-life treatments (those that offer an extension to life and are indicated for small patient populations with a short life expectancy)Citation40. This extended threshold has previously been estimated at ∼£50,000 per QALYCitation41. Brentuximab vedotin meets the SMC criteria for both an ultra-orphan and an end-of-life medicine, as validated by the SMCCitation17. In the present study, probabilistic sensitivity analyses found that, at a willingness-to-pay threshold greater than £50,000, the probability of brentuximab vedotin being cost-effective is 82%, respectively, vs C/R, regardless of whether C/R with intent to alloSCT was included as a comparator.
Prior to the availability of brentuximab vedotin there was no viable treatment option for R/R HL other than to attempt ASCT, with a very low probability of success, or to resort to best supportive careCitation42,Citation43. Evidence for brentuximab vedotin in R/R patients who have not received ASCT is limited to phase I/II studies, a Japanese-only study (TB-BC010088) and Named Patient Programs. Data derived from these studies, based on 40 patients who received the licensed dose, showed an ORR of 54% and a CR rate of 22%Citation44. In addition, 19% of patients went on to receive stem cell transplantation (SCT)Citation44, which can potentially lead to prolonged PFS and cure. This evidence was considered in the SMC recommendation, which includes adult patients with R/R HL following at least two prior therapies when ASCT or multi-agent chemotherapy is not a treatment optionCitation17.
There are a number of limitations to the analysis that largely stem from a lack of comparative data and the heterogeneity of the SG035-0003 population. Ideally, data used in the model would reflect the efficacy in each of the populations in which brentuximab vedotin may be used, namely first-, second-, or subsequent line post-ASCT; and patients eligible/ineligible for alloSCT. However, due to the size of the available population, the model was based on the entire SG035-0003 population (that reflects patients across these strata) and observational data (that follows patients from ASCT relapse or from commencement of a subsequent line of post-ASCT therapy).
The source of efficacy data for brentuximab vedotin (SG035-0003) was a single arm study, which meant that direct or adjusted indirect comparisons were not possible. As such, a naïve indirect comparison was conducted based on the published survival data for the comparators. This may have biased the estimates of clinical outcomes and, hence, cost-effectiveness due to differences in prognostic factors across the trial arms used.
Where patient characteristics were reported consistently across studies enabling a comparison across cohorts for either PFS or OS, there was some observable heterogeneity. In Robinson et al.Citation27, only 80% of patients had previously received ASCT, compared to 100% of patients in SG035-0003, which may have biased PFS in favor of alloSCT. For OS, Martinez et al.Citation9 included higher proportions of patients with Stage III–IV disease at initial diagnosis (52% vs 46%) and with baseline B-symptoms (49% vs 34%) compared to SG035-0003, which may have biased this outcome against C/R and alloSCT. Conversely, SG035-0003 included a higher proportion of patients who were refractory to their most recent therapy (42% vs. 15% in Martinez et al.Citation9).
In addition, Martinez et al.Citation9 did not report characteristics for the alloSCT and C/R cohorts separately; hence, these data include 8% patients who received a second ASCT. Moreover, a number of characteristics were not reported consistently across studies, including; ECOG performance status, Karnofsky status, histologically confirmed CD30-expression, bulky disease, and disease status following frontline or most recent therapy. Consequently, it was not possible to determine the extent of heterogeneity across studies for these characteristics. A multivariate analysis reported by Robinson et al.Citation27 identified poor performance status, defined by Karnofsky score and ECOG score, and disease status at transplant as significant predictors of PFS and OS. Similarly, Martinez et al.Citation9 identified poor performance status, early relapse, stage IV, bulky disease, and age as significant predictors of OS. As such, any heterogeneity in these characteristics would likely bias mean time-in-state and, hence, the cost-effectiveness estimates, increasing uncertainty in the indirect comparison. An attempt was made to adjust for case-mix to reflect the SG035-0003 population; however, it was not possible to adjust PFS for alloSCT, and the risk factor classification used for OS is simplistic. However, any bias may have been conservative, as patients in the Robinson et al.Citation27 study may have had a better prognosis on average than those enrolled in SG035-0003, based on a comparison of median time from ASCT to relapse (9 months in the study by Robinson et al.Citation27 compared with 6.7 months in SG035-0003). Moreover, both the PFS and OS outcomes for brentuximab vedotin used in this analysis reflect survival following the most recent therapyCitation13,Citation15; whereas the self-control data from SG035-0003 reflect PFS following the most recent prior post-ASCT therapy and both of the EBMT studies measured PFS and OS from the time of ASCTCitation9,Citation27. The model can, therefore, be thought of as comparing a mix of first-, second-, and subsequent line post-ASCT patients to less heavily treated patients, which may under-estimate the incremental life-years for brentuximab vedotin. Scenario analyses increasing and decreasing the PFS and OS hazards by 10% for both comparators over lifetime yielded ICERs within the range of those generated by the SMC analysis. These results indicate the potential impact of bias in the relative effects of brentuximab vedotin on PFS and OS on cost-effectiveness, despite not being able to evaluate the full extent of heterogeneity across the studies and the impact of this on clinical outcomes and cost-effectiveness.
The base case analysis extrapolates both PFS and OS for brentuximab vedotin using the hazard for alloSCT and a weighted hazard of alloSCT and C/R, respectively. Extrapolation of the treatment effect observed within the trial periods would have been less conservative; however, this was not pursued given the non-randomized nature of the evidence base.
Although clinicians indicated that brentuximab vedotin followed by alloSCT may be pursued as a treatment option for adequate responders, this comparator was not modeled as only eight patients in the SG035-0003 study received alloSCT as their first treatment after brentuximab vedotin. All of these patients were first-line post-ASCT relapse; representing 8% of all patients included in the trial and 18% of first-line patients included in the trial, and the associated outcomes are captured in the observed OS data used. When including the associated costs for 8% and 18% of patients, the ICER for brentuximab vedotin increased to £45,107 and £53,030 per QALY, respectively. However, patients were censored for progression upon receiving alloSCT in SG035-0003; hence, these results may not fully reflect the health effects of alloSCT and, therefore, the cost-effectiveness of brentuximab vedotin. More recently, Chen et al.Citation45 published extended follow-up from a retrospective analysis comparing outcomes of two consecutive case series; 21 patients who received brentuximab vedotin followed by RIC alloSCT and a historical cohort of 23 patients who received RIC alloSCT in the pre-brentuximab vedotin era. The authors cited these cohorts as being essentially matched aside from their exposure to brentuximab vedotin, and found that salvage brentuximab vedotin yielded an improvement in 2-year PFS. A potential further development of the cost-effectiveness analysis would be to incorporate brentuximab vedotin with intent to alloSCT as a comparator based on the data reported by Chen et al.Citation45.
Due to the relatively early age of presentation compared with many other adult cancers, Hodgkin lymphoma can incur considerable societal costs including loss of productivityCitation46. Incorporating productivity benefits reduced the ICER for brentuximab vedotin to £28,881 per QALY vs C/R. Currently there is no consensus on how to incorporate indirect external effects in cost-effectiveness analyses of healthcare technologies. The human capital approach implemented here may over-estimate the value of productivity by using gross earnings and failing to recognize that additional production may be consumed by the individualCitation47. Furthermore, this method does not reflect the productivity “opportunity costs” of brentuximab vedotin that will occur if brentuximab vedotin is funded (and other productivity-enhancing interventions are therefore displaced). Alternatively, the impact of toxicity on ability to return to work independent of response profile was not modeled; hence, the incremental productivity benefit of brentuximab vedotin may be under-estimated. However, this result highlights the potential impact of incorporating benefits associated with patient’s ability to return to work in this population.
Conclusions
Although the base case ICER for brentuximab vedotin estimated by this analysis exceeds the decision threshold usually applied in Scotland, this result is modest for an orphan medicine that, as recognized by HTA bodies, is unlikely to reach conventional cost-effectiveness thresholdsCitation48. When other considerations are included (such as the ultra-orphan decision-making framework for SMC, or the extended threshold applied by NICE for end-of-life treatments), brentuximab vedotin may still be considered an appropriate use of UK healthcare resources. This is reflected in the positive recommendation from the SMC, based on a previous data cut of SG035-0003, under its decision-making framework for the assessment of ultra-orphan medicines.
Transparency
Declaration of funding
The analysis was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Declaration of financial/other relationships
CP, AB, and JE are employees of ICON plc, an international clinical research organization that has received funding for this work from Millennium Pharmaceuticals Inc. (Cambridge, MA), a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. BW is a former employee of ICON plc. PS is an employee of Millennium Pharmaceuticals Inc. EM and VB were employees of Millennium Pharmaceuticals Inc. at the time of the study. RS and EB are employees of Takeda UK Ltd, Buckinghamshire, UK. AE is an employee of Takeda Pharma AB, Stockholm, Sweden. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Prior presentation
Poster presentation at the American Society of Clinical Oncology Quality Care Symposium, February 26–27, 2016, Phoenix, AZ.
Supplemental_data.doc
Download MS Word (92 KB)Acknowledgments
The authors acknowledge the writing assistance of Fiona Scott of FireKite, an Ashfield company, part of UDG Healthcare plc, during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelinesCitation49.
Note
Notes
1. ADCETRIS is a registered trademark.
References
- Cancer Research UK. Hodgkin lymphoma incidence statistics. Cancer Research UK Cancer statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/hodgkin-lymphoma/incidence. Accessed July 6, 2015
- ISD Scotland. Scottish Cancer Registry. Scottish Cancer Registry Cancer Statistics Hodgkin Lymphoma. http://www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Hodgkins-Disease/. Accessed July 6, 2015
- European Medicines Agency. Public summary of opinion on orphan designation. EU/3/08/596. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2009/10/WC500006354.pdf. Accessed March 17, 2016
- Eichenauer DA, Engert A, Dreyling M. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22(Suppl 6):vi55-8
- Specht L, Gray RG, Clarke MJ, et al. Influence of more extensive radiotherapy and adjuvant chemotherapy on long-term outcome of early-stage Hodgkin's disease: a meta-analysis of 23 randomized trials involving 3,888 patients. International Hodgkin's Disease Collaborative Group. J Clin Oncol 1998;16:830-43
- Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematol Am Soc Hematol Educ Program 2008:326-33
- Arai S, Fanale M, deVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma 2013;54:2531-3
- Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: an international effort. Ann Oncol 2008;19:abstract 118.
- Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin's lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol 2013;24:2430-34
- Seattle Genetics, Inc. ADCETRIS® (brentuximab vedotin) for Injection. Full prescribing information. Food and Drug Administration. http://www.adcetris.com/pdf/ADCETRIS-brentuximab-vedotin-Prescribing-Information.pdf. Accessed July 6, 2015
- Takeda Pharma A/S. ADCETRIS® 50 mg powder for concentrate for solution for infusion. Summary of Product Characteristics. European Medicines Agency. https://www.medicines.org.uk/emc/medicine/27173. Accessed July 6, 2015
- Chen R, Gopal AK, Smith SE, et al. Five-year survival data demonstrating durable responses from a pivotal phase 2 study of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2015;126:2736
- Gopal AK, Chen R, Smith SE, et al. Three-year follow-up data and characterization of long-term remissions from an ongoing phase 2 study of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2013;122:abstract 4382
- Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 2015;125:1236-43
- Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 2012;30:2183-9
- National Institute for Health and Care Excellence. Brentuximab vedotin for treating CD30-positive Hodgkin's lymphoma after autologous stem cell transplant. Proposed Health Technology Appraisal. National Institute for Health and Care Excellence Proposed Health Technology Appraisal. https://www.nice.org.uk/guidance/gid-tag467/documents/lymphoma-hodgkins-cd30positive-brentuximab-vedotin-after-autologous-stem-cell-transplant-draft-scope-for-consultation-prereferral-november-2013-2. Accessed July 6, 2015
- Scottish Medicines Consortium (SMC). Brentuximab vedotin (ADCETRIS®) 50mg powder for concentrate for solution for infusion. SMC No (989/14). https://www.scottishmedicines.org.uk/files/advice/DAD_brentuximab_vedotin__Adcetris__FINAL_Sept_2014_for_website.pdf. Accessed July 6, 2015
- Scottish Medicines Consortium. Guidance to manufacturers for completion of New Product Assessment Form (NPAF). https://www.scottishmedicines.org.uk/Submission_Process/Submission_guidance_and_forms/Templates-Guidance-for-Submission. Accessed July 4, 2016
- Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood 2010;115:3671-7
- Cavalieri E, Matturro A, Annechini G, et al. Efficacy of the BEACOPP regimen in refractory and relapsed Hodgkin lymphoma. Leuk Lymphoma 2009;50:1803-8
- El GT, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol 2007;18:1363-8
- Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378-84
- Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol 2009;146:158-63
- Ng M, Waters J, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone (GEM-P) is an effective salvage regimen in patients with relapsed and refractory lymphoma. Br J Cancer 2005;92:1352-7
- Selby P, Patel P, Milan S, et al. ChlVPP combination chemotherapy for Hodgkin's disease: long-term results. Br J Cancer 1990;62:279-85
- Walewski AJ, Romejko-Jarosinska A, Zwolinski J. DHAP Chemotherapy for recurrent/refractory lymphoma – Five year follow up of 112 patients. Proc Am Soc Clin Oncol 2001;abstract 20
- Robinson SP, Sureda A, Canals C, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: identification of prognostic factors predicting outcome. Haematologica 2009;94:230-8
- Martinez C. Relapse of Hodgkin’s lymphoma after autologous stem cell transplantation (ASCT): Identification of prognostic factors predicting outcome. Oral presented at the 8th International Symposium on Hodgkin Lymphoma, Cologne, Germany, October 23?26, 2010. http://www.informed-scientist.org/presentation/relapse-of-hodgkin-s-lymphoma-after-autologous-stem-cell-transplantation-asct-identification-of-prognostic-factors-predicting-outcome. Accessed July 4, 2016
- Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol 2002;13:1628-35
- Heider A, Niederle N. Efficacy and toxicity of bendamustine in patients with relapsed low-grade non-Hodgkin's lymphomas. Anticancer Drugs 2001;12:725-9
- Diehl V, Sieber M, Ruffer U, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin's disease. The German Hodgkin's Lymphoma Study Group. Ann Oncol 1997;8:143-8
- Joint Formulary Committee. British National Formulary. 67th edn. BMJ Group and Pharmaceutical Press, 2014
- Department of Health NHS England. Reference costs 2012-13. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/261154/nhs_reference_costs_2012-13_acc.pdf. Accessed July 6, 2015
- Swinburn P, Shingler S, Acaster S, et al. Health utilities in relation to treatment response and adverse events in relapsed/refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Leuk Lymphoma 2015;56:1839-45
- Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes 2010;8:50
- Przepiorka D, van Besien K, Khouri I, et al. Carmustine, etoposide, cytarabine and melphalan as a preparative regimen for allogeneic transplantation for high-risk malignant lymphoma. Ann Oncol 1999;10:527-32
- Office for National Statistics. Annual Survey of Hours and Earnings, 2013 Provisional Results. http://www.ons.gov.uk/ons/dcp171778_335027.pdf. Accessed July 6, 2015
- National Institute for Health and Care Excellence (NICE). Pemetrexed for the maintenance treatment of non-small-cell lung cancer. NICE technology appraisal guidance [TA190]. NICE technology appraisal guidance [TA190]. 2010. https://www.nice.org.uk/guidance/ta190. Accessed March 18, 2016
- National Institute for Health and Care Excellence (NICE). Ipilimumab for previously treated advanced (unresectable or metastatic) melanoma. NICE technology appraisal guidance [TA268]. 2012. https://www.nice.org.uk/guidance/ta268. Accessed March 18, 2016
- National Institute for Health and Care Excellence (NICE). Breat cancer (advanced or metastatic – Iapatinib [ID20]. Appraising life-extending, end of life treatments. 2009. https://www.nice.org.uk/guidance/gid-tag387/resources/appraising-life-extending-end-of-life-treatments-paper22009. Accessed July 13, 2015
- Stewart G, Eddowes L, Hamerslag L, et al. The impact of NICE's end-of-life threshold on patient access to new cancer therapies in England And Wales. Value Health 2014;17:A6
- Josting A, Rueffer U, Franklin J, et al. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood 2000;96:1280-6
- Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol 2005;16:625-33
- European Medicines Agency. ADCETRIS® (brentuximab vedotin) European Public Assessment Report (EPAR). Procedure No. EMEA/H/C002455. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002455/WC500135054.pdf. Accessed March 18, 2016
- Chen R, Palmer JM, Tsai NC, et al. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant 2014;20:1864-8
- Hanly P, Soerjomataram I, Sharp L. Measuring the societal burden of cancer: the cost of lost productivity due to premature cancer-related mortality in Europe. Int J Cancer 2015;136:E136-E45
- Claxton K, Walker S, Palmer S, et al. Appropriate perspectives for health care decisions. CHE Research Paper 54. York: University of York, Centre for Health Economics, 2010. http://www.york.ac.uk/che/pdf/rp54.pdf. Accessed March 21, 2016
- Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care 2007;23:36-42
- Battisti WP, Wager E, Baltzer L, et al. Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3. Ann Intern Med 2015;163:461-4